Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Desymmetrization of Trifluoromethylated Tertiary Benzhydrols via Hydrogen-Acceptor-Free Ir-Catalyzed Dehydrogenative C–H Silylation: Decisive Role of the Trifluoromethyl Group
JACS Au ( IF 8.5 ) Pub Date : 2024-02-15 , DOI: 10.1021/jacsau.3c00794 Yoshihiko Yamamoto 1 , Ryu Tadano 1 , Takeshi Yasui 1
JACS Au ( IF 8.5 ) Pub Date : 2024-02-15 , DOI: 10.1021/jacsau.3c00794 Yoshihiko Yamamoto 1 , Ryu Tadano 1 , Takeshi Yasui 1
Affiliation
|
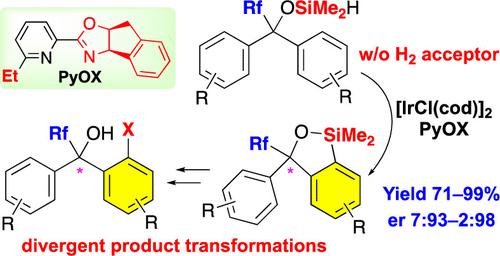
Although the trifluoromethyl (CF3) group is one of the most important fluorinated groups owing to its significant ability to modulate pharmacological properties, constructing trifluoromethylated stereogenic centers in an enantioselective manner has been a formidable challenge. Herein, we report the development of the enantioselective desymmetrization of trifluoromethylated benzhydrols via intramolecular dehydrogenative silylation using Ir catalysts with chiral pyridine-oxazoline (PyOX) ligands. The produced benzoxasilol was transformed into several unsymmetrical benzhydrols via iododesilylation and subsequent transition-metal-catalyzed cross-coupling reactions. Moreover, the same Ir catalyst system was used for the kinetic resolution of unsymmetrical trifluoromethylated benzhydrols.
中文翻译:

通过无氢受体 Ir 催化的脱氢 C–H 硅烷化对三氟甲基化叔苯甲醇进行对映选择性去对称化:三氟甲基的决定性作用
尽管三氟甲基(CF 3 )基团由于其调节药理特性的显着能力而成为最重要的氟化基团之一,但以对映选择性方式构建三氟甲基化立体中心一直是一个艰巨的挑战。在此,我们报道了使用 Ir 催化剂与手性吡啶-恶唑啉 (PyOX) 配体通过分子内脱氢硅烷化对三氟甲基化二苯甲醇进行对映选择性去对称化的进展。通过碘脱甲硅烷基化和随后的过渡金属催化的交叉偶联反应,产生的苯并二硅醇被转化为几种不对称的二苯甲基。此外,相同的 Ir 催化剂体系还用于动力学拆分不对称三氟甲基化二苯甲醇。
更新日期:2024-02-15
中文翻译:

通过无氢受体 Ir 催化的脱氢 C–H 硅烷化对三氟甲基化叔苯甲醇进行对映选择性去对称化:三氟甲基的决定性作用
尽管三氟甲基(CF 3 )基团由于其调节药理特性的显着能力而成为最重要的氟化基团之一,但以对映选择性方式构建三氟甲基化立体中心一直是一个艰巨的挑战。在此,我们报道了使用 Ir 催化剂与手性吡啶-恶唑啉 (PyOX) 配体通过分子内脱氢硅烷化对三氟甲基化二苯甲醇进行对映选择性去对称化的进展。通过碘脱甲硅烷基化和随后的过渡金属催化的交叉偶联反应,产生的苯并二硅醇被转化为几种不对称的二苯甲基。此外,相同的 Ir 催化剂体系还用于动力学拆分不对称三氟甲基化二苯甲醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号