当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Phenol–Pyridinium Salts Enabled by Tandem Electron Donor–Acceptor Complexation and Iridium Photocatalysis
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-16 , DOI: 10.1021/acs.joc.3c02872 Matthew C Carson 1 , Cindy R Liu 1 , Marisa C Kozlowski 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-16 , DOI: 10.1021/acs.joc.3c02872 Matthew C Carson 1 , Cindy R Liu 1 , Marisa C Kozlowski 1
Affiliation
|
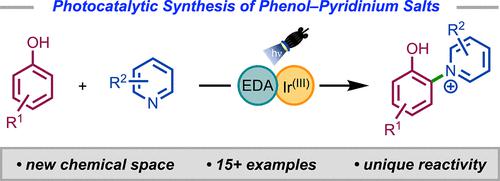
Herein, we describe a dual photocatalytic system to synthesize phenol–pyridinium salts using visible light. Utilizing both electron donor–acceptor (EDA) complex and iridium(III) photocatalytic cycles, the C–N cross-coupling of unprotected phenols and pyridines proceeds in the presence of oxygen to furnish pyridinium salts. Photocatalytic generation of phenoxyl radical cations also enabled a nucleophilic aromatic substitution (SNAr) of a fluorophenol with an electron-poor pyridine. Spectroscopic experiments were conducted to probe the mechanism and reaction selectivity. The unique reactivity of these phenol–pyridinium salts were displayed in several derivatization reactions, providing rapid access to a diverse chemical space.
中文翻译:

串联电子供体-受体络合和铱光催化合成苯酚-吡啶盐
在这里,我们描述了一种使用可见光合成苯酚吡啶鎓盐的双光催化系统。利用电子供体-受体 (EDA) 络合物和铱 (III) 光催化循环,未受保护的酚类和吡啶在氧气存在下发生 C-N 交叉偶联,生成吡啶鎓盐。光催化产生苯氧基自由基阳离子也使得氟苯酚与缺电子吡啶发生亲核芳香取代(S N Ar)。进行光谱实验来探讨其机理和反应选择性。这些苯酚-吡啶鎓盐的独特反应性在几个衍生化反应中表现出来,提供了快速进入不同化学空间的途径。
更新日期:2024-02-16
中文翻译:

串联电子供体-受体络合和铱光催化合成苯酚-吡啶盐
在这里,我们描述了一种使用可见光合成苯酚吡啶鎓盐的双光催化系统。利用电子供体-受体 (EDA) 络合物和铱 (III) 光催化循环,未受保护的酚类和吡啶在氧气存在下发生 C-N 交叉偶联,生成吡啶鎓盐。光催化产生苯氧基自由基阳离子也使得氟苯酚与缺电子吡啶发生亲核芳香取代(S N Ar)。进行光谱实验来探讨其机理和反应选择性。这些苯酚-吡啶鎓盐的独特反应性在几个衍生化反应中表现出来,提供了快速进入不同化学空间的途径。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号