当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sustainable Electrosynthesis of Cyclohexanone Oxime through Nitrate Reduction on a Zn–Cu Alloy Catalyst
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-02-15 , DOI: 10.1021/acscatal.3c05388 Jonathan Sharp 1 , Anna Ciotti 2 , Hayley Andrews 1 , Shaktiswaran R Udayasurian 1 , Max García-Melchor 2 , Tengfei Li 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-02-15 , DOI: 10.1021/acscatal.3c05388 Jonathan Sharp 1 , Anna Ciotti 2 , Hayley Andrews 1 , Shaktiswaran R Udayasurian 1 , Max García-Melchor 2 , Tengfei Li 1
Affiliation
|
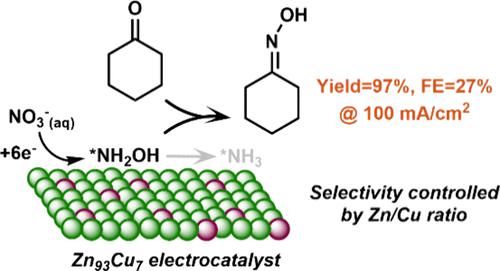
Cyclohexanone oxime is an important precursor for Nylon-6 and is typically synthesized via the nucleophilic addition–elimination of hydroxylamine with cyclohexanone. Current technologies for hydroxylamine production are, however, not environment-friendly due to the requirement of harsh reaction conditions. Here, we report an electrochemical method for the one-pot synthesis of cyclohexanone oxime under ambient conditions with aqueous nitrate as the nitrogen source. A series of Zn–Cu alloy catalysts are developed to drive the electrochemical reduction of nitrate, where the hydroxylamine intermediate formed in the electroreduction process can undergo a chemical reaction with the cyclohexanone present in the electrolyte to produce the corresponding oxime. The best performance is achieved on a Zn93Cu7 electrocatalyst with a 97% yield and a 27% Faradaic efficiency for cyclohexanone oxime at 100 mA/cm2. By analyzing the catalytic activities/selectivities of the different Zn–Cu alloys and conducting in-depth mechanistic studies via in situ Raman spectroscopy and theoretical calculations, we demonstrate that the adsorption of nitrogen species plays a central role in catalytic performance. Overall, this work provides an attractive strategy to build the C–N bond in oxime and drive organic synthesis through electrochemical nitrate reduction, while highlighting the importance of controlling surface adsorption for product selectivity in electrosynthesis.
中文翻译:

锌铜合金催化剂上硝酸盐还原可持续电合成环己酮肟
环己酮肟是 Nylon-6 的重要前体,通常通过羟胺与环己酮的亲核加成-消除反应合成。然而,由于反应条件苛刻,目前的羟胺生产技术并不环保。在这里,我们报道了一种在环境条件下以硝酸水溶液作为氮源一锅合成环己酮肟的电化学方法。开发了一系列锌铜合金催化剂来驱动硝酸盐的电化学还原,其中电解还原过程中形成的羟胺中间体可以与电解质中存在的环己酮发生化学反应,生成相应的肟。在 Zn 93 Cu 7电催化剂上实现了最佳性能,在 100 mA/cm 2下,环己酮肟的产率为 97%,法拉第效率为 27%。通过分析不同锌铜合金的催化活性/选择性,并通过原位拉曼光谱和理论计算进行深入的机理研究,我们证明氮物质的吸附在催化性能中起着核心作用。总体而言,这项工作提供了一种有吸引力的策略,可以在肟中构建 C-N 键并通过电化学硝酸盐还原驱动有机合成,同时强调控制表面吸附对于电合成中产物选择性的重要性。
更新日期:2024-02-15
中文翻译:

锌铜合金催化剂上硝酸盐还原可持续电合成环己酮肟
环己酮肟是 Nylon-6 的重要前体,通常通过羟胺与环己酮的亲核加成-消除反应合成。然而,由于反应条件苛刻,目前的羟胺生产技术并不环保。在这里,我们报道了一种在环境条件下以硝酸水溶液作为氮源一锅合成环己酮肟的电化学方法。开发了一系列锌铜合金催化剂来驱动硝酸盐的电化学还原,其中电解还原过程中形成的羟胺中间体可以与电解质中存在的环己酮发生化学反应,生成相应的肟。在 Zn 93 Cu 7电催化剂上实现了最佳性能,在 100 mA/cm 2下,环己酮肟的产率为 97%,法拉第效率为 27%。通过分析不同锌铜合金的催化活性/选择性,并通过原位拉曼光谱和理论计算进行深入的机理研究,我们证明氮物质的吸附在催化性能中起着核心作用。总体而言,这项工作提供了一种有吸引力的策略,可以在肟中构建 C-N 键并通过电化学硝酸盐还原驱动有机合成,同时强调控制表面吸附对于电合成中产物选择性的重要性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号