当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peroxygenase-Catalyzed Allylic Oxidation Unlocks Telescoped Synthesis of (1S,3R)-3-Hydroxycyclohexanecarbonitrile
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-02-12 , DOI: 10.1021/acscatal.4c00177 Christian M Heckmann 1 , Moritz Bürgler 2 , Caroline E Paul 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-02-12 , DOI: 10.1021/acscatal.4c00177 Christian M Heckmann 1 , Moritz Bürgler 2 , Caroline E Paul 1
Affiliation
|
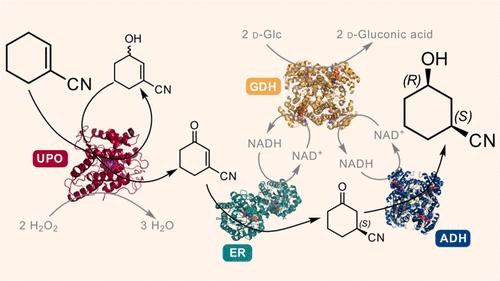
The unmatched chemo-, regio-, and stereoselectivity of enzymes renders them powerful catalysts in the synthesis of chiral active pharmaceutical ingredients (APIs). Inspired by the discovery route toward the LPA1-antagonist BMS-986278, access to the API building block (1S,3R)-3-hydroxycyclohexanecarbonitrile was envisaged using an ene reductase (ER) and alcohol dehydrogenase (ADH) to set both stereocenters. Starting from the commercially available cyclohexene-1-nitrile, a C–H oxyfunctionalization step was required to introduce the ketone functional group, yet several chemical allylic oxidation strategies proved unsuccessful. Enzymatic strategies for allylic oxidation are underdeveloped, with few examples on selected substrates with cytochrome P450s and unspecific peroxygenases (UPOs). In this case, UPOs were found to catalyze the desired allylic oxidation with high chemo- and regioselectivity, at substrate loadings of up to 200 mM, without the addition of organic cosolvents, thus enabling the subsequent ER and ADH steps in a three-step one-pot cascade. UPOs even displayed unreported enantioselective oxyfunctionalization and overoxidation of the substituted cyclohexene. After screening of enzyme panels, the final product was obtained at titers of 85% with 97% ee and 99% de, with a substrate loading of 50 mM, the ER being the limiting step. This synthetic approach provides the first example of a three-step, one-pot UPO-ER-ADH cascade and highlights the potential for UPOs to catalyze diverse enantioselective allylic hydroxylations and oxidations that are otherwise difficult to achieve.
中文翻译:

过氧酶催化烯丙氧化解锁 (1S,3R)-3-羟基环己烷甲腈的大规模合成
酶无与伦比的化学、区域和立体选择性使其成为手性活性药物成分 (API) 合成中的强大催化剂。受 LPA 1拮抗剂 BMS-986278 发现路线的启发,设想使用烯还原酶 (ER) 和乙醇脱氢酶 (ADH) 来获取 API 构建模块 (1 S ,3 R )-3-羟基环己烷甲腈立体中心。从市售的环己烯-1-腈开始,需要一个C-H氧官能化步骤来引入酮官能团,但几种化学烯丙基氧化策略被证明是不成功的。烯丙基氧化的酶促策略尚不成熟,关于细胞色素 P450 和非特异性过氧化酶 (UPO) 的选定底物的例子很少。在这种情况下,UPO 被发现能够在底物负载量高达 200 mM 的情况下以高化学和区域选择性催化所需的烯丙基氧化,无需添加有机共溶剂,从而能够以三步法进行后续的 ER 和 ADH 步骤-锅级联。 UPO 甚至表现出未报道的对映选择性氧官能化和取代环己烯的过度氧化。经过酶组筛选后,最终产物的滴度为 85%, ee为 97%, de 为99%,底物负载量为 50 mM,ER 是限制步骤。这种合成方法提供了三步、一锅UPO-ER-ADH级联的第一个例子,并强调了UPO催化多种对映选择性烯丙基羟基化和氧化反应的潜力,而这些反应是其他方法难以实现的。
更新日期:2024-02-12
中文翻译:

过氧酶催化烯丙氧化解锁 (1S,3R)-3-羟基环己烷甲腈的大规模合成
酶无与伦比的化学、区域和立体选择性使其成为手性活性药物成分 (API) 合成中的强大催化剂。受 LPA 1拮抗剂 BMS-986278 发现路线的启发,设想使用烯还原酶 (ER) 和乙醇脱氢酶 (ADH) 来获取 API 构建模块 (1 S ,3 R )-3-羟基环己烷甲腈立体中心。从市售的环己烯-1-腈开始,需要一个C-H氧官能化步骤来引入酮官能团,但几种化学烯丙基氧化策略被证明是不成功的。烯丙基氧化的酶促策略尚不成熟,关于细胞色素 P450 和非特异性过氧化酶 (UPO) 的选定底物的例子很少。在这种情况下,UPO 被发现能够在底物负载量高达 200 mM 的情况下以高化学和区域选择性催化所需的烯丙基氧化,无需添加有机共溶剂,从而能够以三步法进行后续的 ER 和 ADH 步骤-锅级联。 UPO 甚至表现出未报道的对映选择性氧官能化和取代环己烯的过度氧化。经过酶组筛选后,最终产物的滴度为 85%, ee为 97%, de 为99%,底物负载量为 50 mM,ER 是限制步骤。这种合成方法提供了三步、一锅UPO-ER-ADH级联的第一个例子,并强调了UPO催化多种对映选择性烯丙基羟基化和氧化反应的潜力,而这些反应是其他方法难以实现的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号