当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Production of a Thermostable Mutant of Transglutaminase by Streptomyces mobaraensis
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-02-14 , DOI: 10.1021/acs.jafc.3c07621
Jiacai Ye 1, 2 , Penghui Yang 1, 2 , Jingwen Zhou 1, 2, 3 , Guocheng Du 1, 2 , Song Liu 1, 2, 3
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-02-14 , DOI: 10.1021/acs.jafc.3c07621
Jiacai Ye 1, 2 , Penghui Yang 1, 2 , Jingwen Zhou 1, 2, 3 , Guocheng Du 1, 2 , Song Liu 1, 2, 3
Affiliation
|
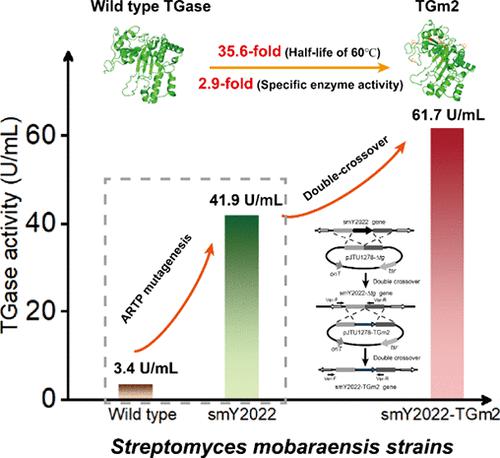
The transglutaminase (TGase) from Streptomyces mobaraensis is widely used to improve the texture of protein-based foods. However, wild-type TGase is not heat-resistant, which is unfavorable for its application. In this study, we successfully constructed a S. mobaraensis strain that can efficiently produce TGm2, a thermostable mutant of S. mobaraensis TGase. First, S. mobaraensis DSM40587 was subjected to atmospheric room temperature plasma mutagenesis, generating mutant smY2022 with a 12.2-fold increase in TGase activity. Then, based on the double-crossover recombination, we replaced the coding sequence of the TGase with that of TGm2 in smY2022, obtaining the strain smY2022-TGm2. The extracellular TGase activity of smY2022-TGm2 reached 61.7 U/mL, 147% higher than that of smY2022. Finally, the catalytic properties of TGm2 were characterized. The half-life time at 60 °C and specific activity of TGm2 reached 64 min and 71.15 U/mg, 35.6- and 2.9-fold higher than those of the wild-type TGase, respectively. As indicated by SDS-PAGE analysis, TGm2 exhibited demonstrably better protein cross-linking ability than the wild-type TGase at 70 °C, although both enzymes shared a similar ability at 40 °C. With improved enzyme production and thermal stability, smY2022-TGm2 could be a competitive strain for the industrial production of transglutaminase.
中文翻译:

利用茂原链霉菌高效生产耐热转谷氨酰胺酶突变体
来自茂原链霉菌的转谷氨酰胺酶 (TGase) 被广泛用于改善蛋白质食品的质地。但野生型TGase不耐热,不利于其应用。在本研究中,我们成功构建了茂原链霉菌TGase的耐热突变体TGm2,该菌株能够高效生产TGm2。首先,对S. mobaraensis DSM40587进行常压室温等离子体诱变,产生TGase活性增加12.2倍的突变体smY2022。然后,基于双交叉重组,将smY2022中TGase的编码序列替换为TGm2的编码序列,得到菌株smY2022-TGm2。 smY2022-TGm2的胞外TGase活性达到61.7 U/mL,比smY2022高147%。最后对TGm2的催化性能进行了表征。 TGm2在60℃下的半衰期和比活性分别达到64分钟和71.15 U/mg,分别是野生型TGase的35.6倍和2.9倍。 SDS-PAGE 分析表明,TGm2 在 70 °C 下表现出比野生型 TGase 更好的蛋白质交联能力,尽管两种酶在 40 °C 下具有相似的能力。 smY2022-TGm2具有改进的酶产量和热稳定性,可能成为转谷氨酰胺酶工业生产的竞争菌株。
更新日期:2024-02-14
中文翻译:

利用茂原链霉菌高效生产耐热转谷氨酰胺酶突变体
来自茂原链霉菌的转谷氨酰胺酶 (TGase) 被广泛用于改善蛋白质食品的质地。但野生型TGase不耐热,不利于其应用。在本研究中,我们成功构建了茂原链霉菌TGase的耐热突变体TGm2,该菌株能够高效生产TGm2。首先,对S. mobaraensis DSM40587进行常压室温等离子体诱变,产生TGase活性增加12.2倍的突变体smY2022。然后,基于双交叉重组,将smY2022中TGase的编码序列替换为TGm2的编码序列,得到菌株smY2022-TGm2。 smY2022-TGm2的胞外TGase活性达到61.7 U/mL,比smY2022高147%。最后对TGm2的催化性能进行了表征。 TGm2在60℃下的半衰期和比活性分别达到64分钟和71.15 U/mg,分别是野生型TGase的35.6倍和2.9倍。 SDS-PAGE 分析表明,TGm2 在 70 °C 下表现出比野生型 TGase 更好的蛋白质交联能力,尽管两种酶在 40 °C 下具有相似的能力。 smY2022-TGm2具有改进的酶产量和热稳定性,可能成为转谷氨酰胺酶工业生产的竞争菌株。

































 京公网安备 11010802027423号
京公网安备 11010802027423号