当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconstitution of Septacidin Biosynthesis in Escherichia coli by Redirecting an ADP-Heptose Precursor from Primary Metabolism
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-02-14 , DOI: 10.1021/acssuschemeng.3c05645 Meng Chen 1, 2 , Min Wang 3 , Zhaoxiang Shi 4 , Pengwei Li 1 , Yuwei Zhang 1 , Zilong Li 1 , Weishan Wang 1, 2 , Yue Tang 1 , Yihua Chen 1, 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2024-02-14 , DOI: 10.1021/acssuschemeng.3c05645 Meng Chen 1, 2 , Min Wang 3 , Zhaoxiang Shi 4 , Pengwei Li 1 , Yuwei Zhang 1 , Zilong Li 1 , Weishan Wang 1, 2 , Yue Tang 1 , Yihua Chen 1, 2
Affiliation
|
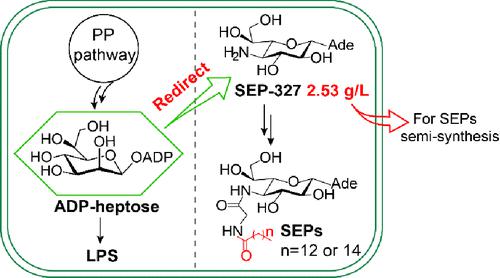
Septacidins (SEPs) represent a group of nucleoside antibiotics featuring an N6-glycosylated l-heptosamine-adenine core (SEP-327), which is challenging for chemical synthesis. The SEP analogues with diverse bioactivities are usually obtained by the long fermentation process of Streptomyces. After delineating the whole biosynthetic pathway of SEPs, we embarked on the reconstitution of SEP and its key intermediate biosynthesis in the fast-growing Escherichia coli cells. We sequentially constructed a set of engineered E. coli strains that can synthesize the N6-glycosylated d-heptose-adenine intermediate (SEP-328), SEP-327, and two SEP congeners with linear fatty acyl groups (SEP-E594 and SEP-E622). For all these constructions, the engineered SEP pathways were shortened significantly by redirecting a key precursor, ADP-l-glycero-β-d-manno-heptose (ADP-heptose), from lipopolysaccharide biosynthesis. The titer of SEP-327 was increased to 470.07 ± 12.81 mg/L at the flask level by improving the solubility of a key oxidase (SepI), supplementing precursors, and optimizing the culture conditions. During fed-batch fermentation, the titer of SEP-327 could reach 2.53 g/L in a 5 L bioreactor using glycerol, a major byproduct of biodiesel industry, as the carbon source, which will significantly facilitate the preparation of SEP-327 for semisynthetic drug development efforts. In addition, we also obtained SEP-E594 and SEP-E622 with a linear fatty acyl terminus that exhibits antifungal activities comparable to those of their congeners with a branched fatty acyl group.
中文翻译:

通过从初级代谢中重定向 ADP-庚糖前体来重建大肠杆菌中的 Septacidin 生物合成
Septacidins (SEP) 代表一组以N 6 -糖基化l -七糖胺腺嘌呤核心 (SEP-327) 为特征的核苷抗生素,这对于化学合成来说具有挑战性。具有多种生物活性的SEP类似物通常是通过链霉菌的长期发酵过程获得的。在描述了 SEP 的整个生物合成途径后,我们开始在快速生长的大肠杆菌细胞中重建 SEP 及其关键的中间生物合成。我们依次构建了一组工程大肠杆菌菌株,可以合成N 6 -糖基化d-庚糖-腺嘌呤中间体(SEP-328)、SEP-327和两个具有线性脂肪酰基的SEP同源物(SEP-E594和SEP) -E622)。对于所有这些构建,通过重定向来自脂多糖生物合成的关键前体ADP - 1-甘油-β- d-甘露-庚糖(ADP-庚糖),工程化的SEP途径显着缩短。通过提高关键氧化酶(SepI)的溶解度、补充前体以及优化培养条件,SEP-327在烧瓶水平上的效价提高至470.07±12.81mg/L。在补料分批发酵过程中,以生物柴油工业的主要副产品甘油为碳源,在5 L生物反应器中,SEP-327的效价可达到2.53 g/L,这将极大地促进SEP-327半合成的制备药物开发工作。此外,我们还获得了具有线性脂肪酰基末端的SEP-E594和SEP-E622,其表现出与具有支链脂肪酰基的同类物相当的抗真菌活性。
更新日期:2024-02-14
中文翻译:

通过从初级代谢中重定向 ADP-庚糖前体来重建大肠杆菌中的 Septacidin 生物合成
Septacidins (SEP) 代表一组以N 6 -糖基化l -七糖胺腺嘌呤核心 (SEP-327) 为特征的核苷抗生素,这对于化学合成来说具有挑战性。具有多种生物活性的SEP类似物通常是通过链霉菌的长期发酵过程获得的。在描述了 SEP 的整个生物合成途径后,我们开始在快速生长的大肠杆菌细胞中重建 SEP 及其关键的中间生物合成。我们依次构建了一组工程大肠杆菌菌株,可以合成N 6 -糖基化d-庚糖-腺嘌呤中间体(SEP-328)、SEP-327和两个具有线性脂肪酰基的SEP同源物(SEP-E594和SEP) -E622)。对于所有这些构建,通过重定向来自脂多糖生物合成的关键前体ADP - 1-甘油-β- d-甘露-庚糖(ADP-庚糖),工程化的SEP途径显着缩短。通过提高关键氧化酶(SepI)的溶解度、补充前体以及优化培养条件,SEP-327在烧瓶水平上的效价提高至470.07±12.81mg/L。在补料分批发酵过程中,以生物柴油工业的主要副产品甘油为碳源,在5 L生物反应器中,SEP-327的效价可达到2.53 g/L,这将极大地促进SEP-327半合成的制备药物开发工作。此外,我们还获得了具有线性脂肪酰基末端的SEP-E594和SEP-E622,其表现出与具有支链脂肪酰基的同类物相当的抗真菌活性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号