当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
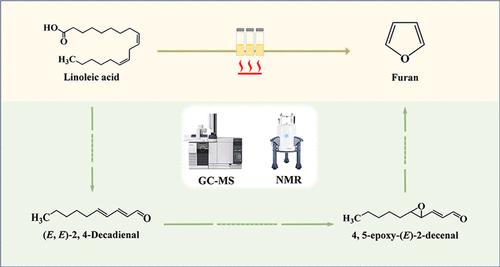
Formation of Furan from Linoleic Acid Thermal Oxidation: (E,E)-2,4-Decadienal as a Critical Intermediate Product
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-02-14 , DOI: 10.1021/acs.jafc.3c08604 Qing Zhang 1, 2 , Jiaping Ke 1, 2 , Piaopiao Long 1, 2 , Mingchun Wen 1, 2 , Zisheng Han 1, 2 , Liang Zhang 1, 2 , Mengting Zhu 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-02-14 , DOI: 10.1021/acs.jafc.3c08604 Qing Zhang 1, 2 , Jiaping Ke 1, 2 , Piaopiao Long 1, 2 , Mingchun Wen 1, 2 , Zisheng Han 1, 2 , Liang Zhang 1, 2 , Mengting Zhu 1, 2
Affiliation
|
The linoleic acid reaction models were set at 150 °C for 120 min, and its oxidation process was monitored by nuclear magnetic resonance (NMR) and gas chromatography–mass spectrometry (GC-MS). Results showed that no furan was formed from linoleic acid without heating, while furan accumulated throughout the heating process. Linoleic acid ran out within 30 min, which indicated that furan was formed mainly from the intermediate oxidation products of linoleic acid after 30 min. It should be noticed that the content of (E,E)-2,4-decadienal reached maximum once the linoleic acid ran out and then decreased with the formation of furan. Multivariate statistical analysis suggested that (E,E)-2,4-decadienal was the most important aldehyde related to furan formation during linoleic acid oxidation. To prove this assumption, the variation of furan from (E,E)-2,4-decadienal reaction models heating at 150 °C for 60 min was also studied. Results showed that the content of furan increased with the oxidation of (E,E)-2,4-decadienal. Furthermore, NMR and GC-MS data proved that (E,E)-2,4-decadienal could be oxidized to 4,5-epoxy-(E)-2-decenal. In conclusion, our results supported (E,E)-2,4-decadienal and trans-4,5-epoxy-(E)-2-decenal as critical intermediate products of furan formation from linoleic acid oxidation.
中文翻译:

亚油酸热氧化形成呋喃:(E,E)-2,4-癸二烯醛作为关键中间产物
亚油酸反应模型设定在150℃,120分钟,并通过核磁共振(NMR)和气相色谱-质谱(GC-MS)监测其氧化过程。结果表明,在不加热的情况下,亚油酸不会形成呋喃,而在整个加热过程中呋喃不断积累。亚油酸在30分钟内耗尽,这表明30分钟后呋喃主要由亚油酸的中间氧化产物形成。值得注意的是,当亚油酸耗尽时, ( E,E )-2,4-癸二烯醛的含量达到最大值,然后随着呋喃的形成而降低。多变量统计分析表明( E , E )-2,4-癸二醛是与亚油酸氧化过程中呋喃形成相关的最重要的醛。为了证明这一假设,还研究了 ( E,E )-2,4-癸二烯醛反应模型在 150 °C 加热 60 分钟时呋喃的变化。结果表明,随着( E , E )-2,4-癸二烯醛的氧化,呋喃含量增加。此外,NMR和GC-MS数据证明( E , E )-2,4-癸烯醛可以被氧化为4,5-环氧-( E )-2-癸烯醛。总之,我们的结果支持( E , E )-2,4-癸二烯醛和反式-4,5-环氧-( E )-2-癸烯醛是亚油酸氧化形成呋喃的关键中间产物。
更新日期:2024-02-14
中文翻译:

亚油酸热氧化形成呋喃:(E,E)-2,4-癸二烯醛作为关键中间产物
亚油酸反应模型设定在150℃,120分钟,并通过核磁共振(NMR)和气相色谱-质谱(GC-MS)监测其氧化过程。结果表明,在不加热的情况下,亚油酸不会形成呋喃,而在整个加热过程中呋喃不断积累。亚油酸在30分钟内耗尽,这表明30分钟后呋喃主要由亚油酸的中间氧化产物形成。值得注意的是,当亚油酸耗尽时, ( E,E )-2,4-癸二烯醛的含量达到最大值,然后随着呋喃的形成而降低。多变量统计分析表明( E , E )-2,4-癸二醛是与亚油酸氧化过程中呋喃形成相关的最重要的醛。为了证明这一假设,还研究了 ( E,E )-2,4-癸二烯醛反应模型在 150 °C 加热 60 分钟时呋喃的变化。结果表明,随着( E , E )-2,4-癸二烯醛的氧化,呋喃含量增加。此外,NMR和GC-MS数据证明( E , E )-2,4-癸烯醛可以被氧化为4,5-环氧-( E )-2-癸烯醛。总之,我们的结果支持( E , E )-2,4-癸二烯醛和反式-4,5-环氧-( E )-2-癸烯醛是亚油酸氧化形成呋喃的关键中间产物。







































 京公网安备 11010802027423号
京公网安备 11010802027423号