当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of gem-Difluoro-3,4-dihydro-2H-pyrans via a TfOH-Catalyzed [4 + 2] Annulation of Difluoroenoxysilanes with α-Cyano Chalcones
Organic Letters ( IF 4.9 ) Pub Date : 2024-02-14 , DOI: 10.1021/acs.orglett.4c00078 Jing Zhang 1 , Daokai Xiong 1 , Zhiwei Jiang 1 , Shuaiting Chen 1 , Guo-Bo Huang 1 , Jinshan Li 2 , Zhiming Wang 1 , Jianguo Yang 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-02-14 , DOI: 10.1021/acs.orglett.4c00078 Jing Zhang 1 , Daokai Xiong 1 , Zhiwei Jiang 1 , Shuaiting Chen 1 , Guo-Bo Huang 1 , Jinshan Li 2 , Zhiming Wang 1 , Jianguo Yang 1
Affiliation
|
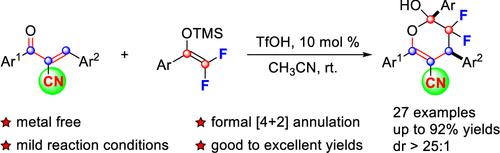
Difluoroenoxysilane, a commonly used difluoroallylating reagent, has attracted considerable attention in recent years. However, its application in the annulation reaction for the construction of fluorinated heterocyclic compounds remains relatively limited. Presented here is the Brønsted acid-catalyzed efficient formal [4 + 2] annulation of difluoroenoxysilanes with α-cyano chalcones. The developed protocol demonstrates tolerance to various substituents under mild reaction conditions, providing a reliable approach to construct gem-difluoro-3,4-dihydro-2H-pyrans in good to excellent yields with high diastereoselectivities.
中文翻译:

通过 TfOH 催化的二氟烯氧基硅烷与 α-氰基查耳酮的 [4 + 2] 环化合成偕二氟-3,4-二氢-2H-吡喃
二氟烯氧基硅烷是一种常用的二氟烯氧基化试剂,近年来引起了人们的广泛关注。然而,其在构建氟化杂环化合物的成环反应中的应用仍然相对有限。这里介绍的是二氟烯氧基硅烷与 α-氰基查耳酮在布朗斯台德酸催化下的高效形式 [4 + 2] 成环反应。所开发的方案证明了在温和反应条件下对各种取代基的耐受性,提供了一种可靠的方法来构建宝石-二氟-3,4-二氢-2 H-吡喃,以良好至优异的产率和高非对映选择性。
更新日期:2024-02-14
中文翻译:

通过 TfOH 催化的二氟烯氧基硅烷与 α-氰基查耳酮的 [4 + 2] 环化合成偕二氟-3,4-二氢-2H-吡喃
二氟烯氧基硅烷是一种常用的二氟烯氧基化试剂,近年来引起了人们的广泛关注。然而,其在构建氟化杂环化合物的成环反应中的应用仍然相对有限。这里介绍的是二氟烯氧基硅烷与 α-氰基查耳酮在布朗斯台德酸催化下的高效形式 [4 + 2] 成环反应。所开发的方案证明了在温和反应条件下对各种取代基的耐受性,提供了一种可靠的方法来构建宝石-二氟-3,4-二氢-2 H-吡喃,以良好至优异的产率和高非对映选择性。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号