Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Synthesis of 3,4-Dihydrobenzofuro[3,2-b]pyridin-2(1H)-ones Enabled by Pd/Chiral Isothiourea Relay Catalysis
Synthesis ( IF 2.2 ) Pub Date : 2024-02-13 , DOI: 10.1055/s-0043-1763679 Mostafa Sayed 1, 2 , Zhi-Yong Han 1 , Zhipeng Shi 1 , Tao Fan 1 , Hong-Cheng Shen 1
中文翻译:

Pd/手性异硫脲中继催化立体选择性合成 3,4-二氢苯并呋喃[3,2-b]吡啶-2(1H)-酮
更新日期:2024-02-14
Synthesis ( IF 2.2 ) Pub Date : 2024-02-13 , DOI: 10.1055/s-0043-1763679 Mostafa Sayed 1, 2 , Zhi-Yong Han 1 , Zhipeng Shi 1 , Tao Fan 1 , Hong-Cheng Shen 1
Affiliation
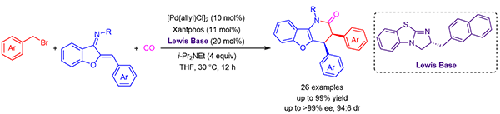
|
A highly enantioselective [4+2] cyclization of azadienes with ketene in situ generated from Pd-catalyzed carbonylation of benzyl bromides, is established through Pd/chiral isothiourea relay catalysis. The key in this transformation is the formation of a C1-ammonium enolate from the in situ generated ketene and a chiral isothiourea catalyst, which subsequently undergoes a formal [4+2] reaction, leading to 3,4-dihydrobenzofuro[3,2-b]pyridine derivatives in high yields and excellent levels of stereoselectivity.
中文翻译:

Pd/手性异硫脲中继催化立体选择性合成 3,4-二氢苯并呋喃[3,2-b]吡啶-2(1H)-酮
通过 Pd/手性异硫脲中继催化,建立了氮杂二烯与乙烯酮的高度对映选择性 [4+2] 环化,该乙烯酮是由 Pd 催化的苄基溴的羰基化反应产生的。该转化的关键是从原位生成的乙烯酮和手性异硫脲催化剂形成C1-烯醇铵,随后进行正式的[4+2]反应,生成3,4-二氢苯并呋喃[3,2- b ]吡啶衍生物具有高产率和优异的立体选择性水平。































 京公网安备 11010802027423号
京公网安备 11010802027423号