当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of the Novel, Orally Active, and Selective LPA1 Receptor Antagonist ACT-1016-0707 as a Preclinical Candidate for the Treatment of Fibrotic Diseases
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-02-13 , DOI: 10.1021/acs.jmedchem.3c01827 Cyrille Lescop 1 , Magdalena Birker 2 , Christine Brotschi 1 , Cédric Bürki 3 , Keith Morrison 4 , Sylvie Froidevaux 4 , Stéphane Delahaye 5 , Oliver Nayler 2 , Martin H Bolli 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-02-13 , DOI: 10.1021/acs.jmedchem.3c01827 Cyrille Lescop 1 , Magdalena Birker 2 , Christine Brotschi 1 , Cédric Bürki 3 , Keith Morrison 4 , Sylvie Froidevaux 4 , Stéphane Delahaye 5 , Oliver Nayler 2 , Martin H Bolli 1
Affiliation
|
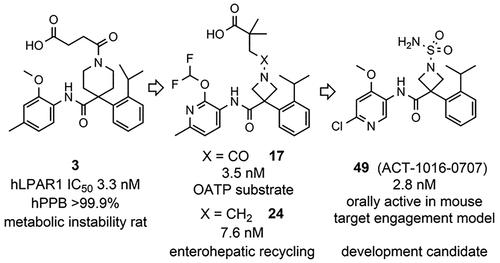
Piperidine 3 is a potent and selective lysophosphatidic acid receptor subtype 1 receptor (LPAR1) antagonist that has shown efficacy in a skin vascular leakage target engagement model in mice. However, compound 3 has very high human plasma protein binding and high clearance in rats, which could significantly hamper its clinical development. Continued lead optimization led to the potent, less protein bound, metabolically stable, and orally active azetidine 17. Rat pharmacokinetics (PK) studies revealed that 17 accumulated in the liver. In vitro studies indicated that 17 is an organic anion co-transporting polypeptide 1B1 (OATP1B1) substrate. Although analogue 24 was no longer a substrate of OATP1B1, PK studies suggested that the compound undergoes enterohepatic recirculation. Replacing the carboxylic acidic side chain by a non-acidic sulfamide moiety and further fine-tuning of the scaffold yielded the potent, orally active LPAR1 antagonist 49, which was selected for preclinical development for the treatment of fibrotic diseases.
中文翻译:

发现新型、口服活性和选择性 LPA1 受体拮抗剂 ACT-1016-0707 作为治疗纤维化疾病的临床前候选药物
哌啶 3 是一种有效的选择性溶血磷脂酸受体亚型 1 受体 (LPAR1) 拮抗剂,已在小鼠的皮肤血管渗漏靶标参与模型中显示出疗效。然而,化合物 3 在大鼠中具有非常高的人血浆蛋白结合率和高清除率,这可能会严重阻碍其临床开发。持续的先导化合物优化导致有效、蛋白质结合较少、代谢稳定且具有口服活性的阿兹替丁 17。大鼠药代动力学 (PK) 研究表明,17 种在肝脏中积累。体外研究表明,17 是一种有机阴离子共转运多肽 1B1 (OATP1B1) 底物。尽管类似物 24 不再是 OATP1B1 的底物,但 PK 研究表明该化合物经历了肠肝再循环。用非酸性磺胺部分取代羧酸性侧链并进一步微调支架产生了有效的口服活性 LPAR1 拮抗剂 49,该拮抗剂被选为治疗纤维化疾病的临床前开发。
更新日期:2024-02-13
中文翻译:

发现新型、口服活性和选择性 LPA1 受体拮抗剂 ACT-1016-0707 作为治疗纤维化疾病的临床前候选药物
哌啶 3 是一种有效的选择性溶血磷脂酸受体亚型 1 受体 (LPAR1) 拮抗剂,已在小鼠的皮肤血管渗漏靶标参与模型中显示出疗效。然而,化合物 3 在大鼠中具有非常高的人血浆蛋白结合率和高清除率,这可能会严重阻碍其临床开发。持续的先导化合物优化导致有效、蛋白质结合较少、代谢稳定且具有口服活性的阿兹替丁 17。大鼠药代动力学 (PK) 研究表明,17 种在肝脏中积累。体外研究表明,17 是一种有机阴离子共转运多肽 1B1 (OATP1B1) 底物。尽管类似物 24 不再是 OATP1B1 的底物,但 PK 研究表明该化合物经历了肠肝再循环。用非酸性磺胺部分取代羧酸性侧链并进一步微调支架产生了有效的口服活性 LPAR1 拮抗剂 49,该拮抗剂被选为治疗纤维化疾病的临床前开发。













































 京公网安备 11010802027423号
京公网安备 11010802027423号