当前位置:
X-MOL 学术
›
J. Agric. Food Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Interaction Mechanism of Picolinamide Fungicide Targeting on the Cytochrome bc1 Complex and Its Structural Modification
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-02-12 , DOI: 10.1021/acs.jafc.3c05982
Ying Dong 1 , Bo Li 1 , Mao-Xue Yin 1 , Zheng Liu 1 , Yan Niu 1 , Qiong-You Wu 1 , Xiao-Lei Zhu 1 , Guang-Fu Yang 1, 2
Journal of Agricultural and Food Chemistry ( IF 5.7 ) Pub Date : 2024-02-12 , DOI: 10.1021/acs.jafc.3c05982
Ying Dong 1 , Bo Li 1 , Mao-Xue Yin 1 , Zheng Liu 1 , Yan Niu 1 , Qiong-You Wu 1 , Xiao-Lei Zhu 1 , Guang-Fu Yang 1, 2
Affiliation
|
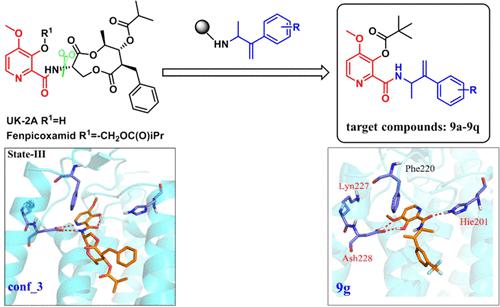
Picolinamide fungicides, structurally related to UK-2A and antimycin-A, bind into the Qi-site in the bc1 complex. However, the detailed binding mode of picolinamide fungicides remains unknown. In the present study, antimycin-A and UK-2A were selected to study the binding mode of picolinamide inhibitors with four protonation states in the Qi-site by integrating molecular dynamics simulation, molecular docking, and molecular mechanics Generalized Born surface area (MM/GBSA) calculations. Subsequently, a series of new picolinamide derivatives were designed and synthesized to further understand the effects of substituents on the tail phenyl ring. The computational results indicated that the substituted aromatic rings in antimycin-A and UK-2A were the pharmacophore fragments and made the primary contribution when bound to a protein. Compound 9g-hydrolysis formed H-bonds with Hie201 and Ash228 and showed an IC50 value of 6.05 ± 0.24 μM against the porcine bc1 complex. Compound 9c, with a simpler chemical structure, showed higher control effects than florylpicoxamid against cucumber downy mildew and expanded the fungicidal spectrum of picolinamide fungicides. The structural and mechanistic insights obtained from the present study will provide a valuable clue for the future designing of new promising Qi-site inhibitors.
中文翻译:

吡啶甲酰胺类杀菌剂针对细胞色素bc1复合物的相互作用机制及其结构修饰
吡啶酰胺类杀菌剂在结构上与 UK-2A 和抗霉素-A 相关,结合到bc 1复合物中的 Qi 位点。然而,吡啶甲酰胺类杀菌剂的详细结合模式仍然未知。本研究选择抗霉素-A和UK-2A,通过结合分子动力学模拟、分子对接和分子力学,研究Qi位点四种质子化态的吡啶甲酰胺抑制剂的结合模式。 GBSA)计算。随后,设计合成了一系列新型吡啶酰胺衍生物,以进一步了解取代基对尾苯环的影响。计算结果表明,抗霉素-A和UK-2A中的取代芳环是药效团片段,在与蛋白质结合时起主要作用。化合物9g 水解与 Hie201 和 Ash228 形成氢键,并且针对猪bc 1复合物显示出 6.05 ± 0.24 μM 的 IC 50值。化合物9c化学结构更简单,对黄瓜霜霉病的防治效果优于氟啶酰胺,扩大了吡啶甲酰胺类杀菌剂的杀菌谱。从本研究中获得的结构和机制见解将为未来设计新型有前景的 Qi 位点抑制剂提供宝贵的线索。
更新日期:2024-02-12
中文翻译:

吡啶甲酰胺类杀菌剂针对细胞色素bc1复合物的相互作用机制及其结构修饰
吡啶酰胺类杀菌剂在结构上与 UK-2A 和抗霉素-A 相关,结合到bc 1复合物中的 Qi 位点。然而,吡啶甲酰胺类杀菌剂的详细结合模式仍然未知。本研究选择抗霉素-A和UK-2A,通过结合分子动力学模拟、分子对接和分子力学,研究Qi位点四种质子化态的吡啶甲酰胺抑制剂的结合模式。 GBSA)计算。随后,设计合成了一系列新型吡啶酰胺衍生物,以进一步了解取代基对尾苯环的影响。计算结果表明,抗霉素-A和UK-2A中的取代芳环是药效团片段,在与蛋白质结合时起主要作用。化合物9g 水解与 Hie201 和 Ash228 形成氢键,并且针对猪bc 1复合物显示出 6.05 ± 0.24 μM 的 IC 50值。化合物9c化学结构更简单,对黄瓜霜霉病的防治效果优于氟啶酰胺,扩大了吡啶甲酰胺类杀菌剂的杀菌谱。从本研究中获得的结构和机制见解将为未来设计新型有前景的 Qi 位点抑制剂提供宝贵的线索。




































 京公网安备 11010802027423号
京公网安备 11010802027423号