当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Difluoromethylated Difunctionalization of Alkenes under Visible Light
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-01 , DOI: 10.1021/acs.joc.3c02552 Yuping Zhu 1 , Yan-Hua Qiu 1 , Xiao-Kang Dai 1 , Wenjun Luo 1 , Xiangjun Peng 2 , Zhengwang Chen 1 , Daohong Yu 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-02-01 , DOI: 10.1021/acs.joc.3c02552 Yuping Zhu 1 , Yan-Hua Qiu 1 , Xiao-Kang Dai 1 , Wenjun Luo 1 , Xiangjun Peng 2 , Zhengwang Chen 1 , Daohong Yu 1
Affiliation
|
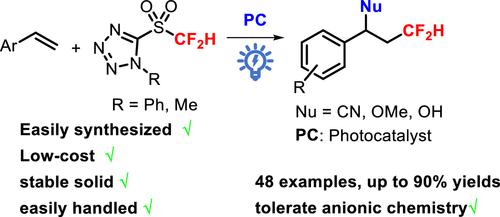
Difluoromethylated compounds usually act as bioisosteres for alcohol functional groups and show unique physicochemical and biological properties. The cyano-difluoromethylation of alkenes using 5-((difluoromethyl)sulfonyl)-1-phenyl-1H-tetrazole as a CF2H radical difluoromethyl precursor was developed to afford nitriles including a CF2H group. A low-cost, stable, easily handled 5-((difluoromethyl)sulfonyl)-1-methyl-1H-tetrazole (DFSMT) was synthesized and applied as the radical CF2H reagent. Using DFSMT as the radical CF2H precursor, the oxyl-difluoromethylation of alkenes was developed to obtain difluoromethylated ether products. All of the reactions showed good functional group tolerability. Initial mechanistic experiments indicated that the CF2H radical was involved as the key active intermediate.
中文翻译:

可见光下烯烃的二氟甲基化双官能化
二氟甲基化化合物通常充当醇官能团的生物等排体,并表现出独特的理化和生物学特性。使用5-((二氟甲基)磺酰基)-1-苯基-1H-四唑作为CF 2 H自由基二氟甲基前体进行烯烃的氰基二氟甲基化,得到包含CF 2 H基团的腈。合成了低成本、稳定、易于操作的5-((二氟甲基)磺酰基)-1-甲基-1H-四唑(DFSMT),并将其用作自由基CF 2 H试剂。使用DFSMT作为自由基CF 2 H前体,开发了烯烃的氧基二氟甲基化反应以获得二氟甲基化醚产物。所有反应均表现出良好的官能团耐受性。初步的机理实验表明CF 2 H自由基作为关键的活性中间体参与其中。
更新日期:2024-02-01
中文翻译:

可见光下烯烃的二氟甲基化双官能化
二氟甲基化化合物通常充当醇官能团的生物等排体,并表现出独特的理化和生物学特性。使用5-((二氟甲基)磺酰基)-1-苯基-1H-四唑作为CF 2 H自由基二氟甲基前体进行烯烃的氰基二氟甲基化,得到包含CF 2 H基团的腈。合成了低成本、稳定、易于操作的5-((二氟甲基)磺酰基)-1-甲基-1H-四唑(DFSMT),并将其用作自由基CF 2 H试剂。使用DFSMT作为自由基CF 2 H前体,开发了烯烃的氧基二氟甲基化反应以获得二氟甲基化醚产物。所有反应均表现出良好的官能团耐受性。初步的机理实验表明CF 2 H自由基作为关键的活性中间体参与其中。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号