当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scalable Synthesis of C5aR1 Antagonist ACT-1014-6470 via N7-Selective Reductive Amination of an Unprotected Pyrazole Starting Material and Intramolecular Urea Formation with 1,1′-Carbonyl-di(1,2,4-triazol) (CDT)
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-02-08 , DOI: 10.1021/acs.oprd.3c00492 Stefan Reber 1 , Nicole Blumer 1 , Daniel Leuenberger 1 , Tony Fleischer 1 , Dorte Renneberg 1 , Stefan Abele 1 , Gabriel Schäfer 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-02-08 , DOI: 10.1021/acs.oprd.3c00492 Stefan Reber 1 , Nicole Blumer 1 , Daniel Leuenberger 1 , Tony Fleischer 1 , Dorte Renneberg 1 , Stefan Abele 1 , Gabriel Schäfer 1
Affiliation
|
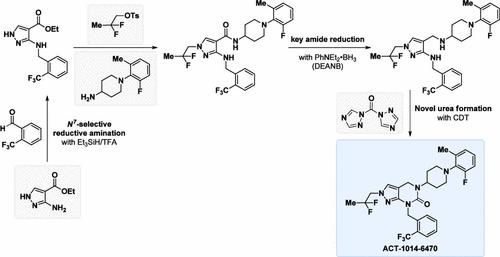
ACT-1014-6470 is a potent, orally available, reversible, and selective C5aR1 antagonist. Herein, we report the development of a scalable and robust process for the preparation of ACT-1014-6470 on kg scale. The synthetic sequence started from two inexpensive starting materials─ethyl 3-amino-1H-pyrazole-4-carboxylate and 2-(trifluoromethyl)benzaldehyde─which were coupled together with a novel reductive amination protocol for electron-poor heterocycles that was perfectly N7-selective. After building up the core API-structure via a sequence of N2-pyrazole alkylation, ester hydrolysis, amide formation, and reduction, the final intramolecular urea formation was performed with a novel protocol using 1,1′-carbonyl-di(1,2,4-triazol), CDT. The cyclization worked under mild conditions at room temperature without the need of additional base and provided the API in high purity (99.4% a/a by HPLC, 99% w/w) after aqueous workup and crystallization from EtOH. In total, over 2.5 kg of ACT-1014-6470 were prepared in-house using the described 11-step synthesis, with the longest linear sequence (6 steps) having an overall yield of 42%.
中文翻译:

通过未受保护的吡唑起始原料的 N7 选择性还原胺化以及与 1,1'-羰基-二(1,2,4-三唑) (CDT) 形成分子内脲来大规模合成 C5aR1 拮抗剂 ACT-1014-6470
ACT-1014-6470 是一种有效的、口服的、可逆的、选择性的 C5aR1 拮抗剂。在此,我们报告了用于制备公斤级 ACT-1014-6470 的可扩展且稳健的工艺的开发。该合成序列从两种廉价的起始材料开始——3-氨基-1H-吡唑-4-甲酸乙酯和2-(三氟甲基)苯甲醛——与一种新型的贫电子杂环还原胺化方案结合在一起,该方案完全N < b0> -选择性。通过一系列 N 2 -吡唑烷基化、酯水解、酰胺形成和还原构建核心 API 结构后,使用 1,1'- 的新方案进行最终的分子内尿素形成羰基二(1,2,4-三唑),CDT。环化在室温温和条件下进行,无需额外的碱,并在水性后处理和从 EtOH 中结晶后提供高纯度的 API(HPLC 检测为 99.4% a/a,99% w/w)。总共超过 2.5 公斤的 ACT-1014-6470 使用所述的 11 步合成在内部制备,其中最长的线性序列(6 步)的总产率为 42%。
更新日期:2024-02-08
中文翻译:

通过未受保护的吡唑起始原料的 N7 选择性还原胺化以及与 1,1'-羰基-二(1,2,4-三唑) (CDT) 形成分子内脲来大规模合成 C5aR1 拮抗剂 ACT-1014-6470
ACT-1014-6470 是一种有效的、口服的、可逆的、选择性的 C5aR1 拮抗剂。在此,我们报告了用于制备公斤级 ACT-1014-6470 的可扩展且稳健的工艺的开发。该合成序列从两种廉价的起始材料开始——3-氨基-1H-吡唑-4-甲酸乙酯和2-(三氟甲基)苯甲醛——与一种新型的贫电子杂环还原胺化方案结合在一起,该方案完全N < b0> -选择性。通过一系列 N 2 -吡唑烷基化、酯水解、酰胺形成和还原构建核心 API 结构后,使用 1,1'- 的新方案进行最终的分子内尿素形成羰基二(1,2,4-三唑),CDT。环化在室温温和条件下进行,无需额外的碱,并在水性后处理和从 EtOH 中结晶后提供高纯度的 API(HPLC 检测为 99.4% a/a,99% w/w)。总共超过 2.5 公斤的 ACT-1014-6470 使用所述的 11 步合成在内部制备,其中最长的线性序列(6 步)的总产率为 42%。































 京公网安备 11010802027423号
京公网安备 11010802027423号