当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dehydrogenation and Transfer Hydrogenation of Alkenones to Phenols and Ketones on Carbon-Supported Noble Metals
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-02-09 , DOI: 10.1021/acscatal.3c04849 Katja Li 1 , H Ray Kelly 2 , Ana Franco 3 , Victor S Batista 2 , Eszter Baráth 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-02-09 , DOI: 10.1021/acscatal.3c04849 Katja Li 1 , H Ray Kelly 2 , Ana Franco 3 , Victor S Batista 2 , Eszter Baráth 1, 3
Affiliation
|
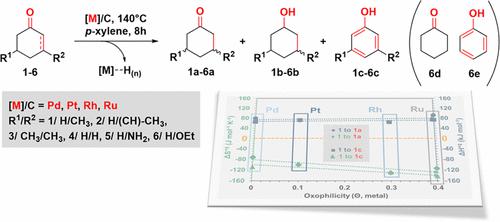
The catalytic dehydrogenation of substituted alkenones on noble metal catalysts supported on carbon (Pt/C, Pd/C, Rh/C, and Ru/C) was investigated in an organic phase under inert conditions. The dehydrogenation and semihydrogenation of the enone starting materials resulted in aromatic compounds (primary products), saturated cyclic ketones (secondary products), and cyclic alcohols (minor products). Pd/C exhibits the highest catalytic activity, followed by Pt/C and Rh/C. Aromatic compounds remain the primary products, even in the presence of hydrogen donors. Joint experimental and theoretical analyses showed that the four catalytic materials stabilize a common dienol intermediate on the metal surfaces, formed by keto–enol tautomerization. This intermediate subsequently forms aromatic products upon dehydrogenation. The binding orientation of the enone reactants on the catalytic surface is strongly metal-dependent, as the M–O bond distance changes substantially according to the metal. The longer M–O bonds (Pt: 2.84 Å > Pd: 2.23 Å > Rh: 2.17 Å > Ru: 2.07 Å) correlate with faster reaction rates and more favorable keto–enol tautomerization, as shorter distances correspond to a more stabilized starting material. Tautomerization is shown to occur via a stepwise surface-assisted pathway. Overall, each of the studied metals exhibits a distinct balance of enthalpy and entropy of activation (ΔH°‡, ΔS°‡), offering unique possibilities in the realm of enone dehydrogenation reactions that can be achieved by suitable selection of catalytic materials.
中文翻译:

碳载贵金属上烯酮脱氢和转移加氢生成酚和酮
在惰性条件下的有机相中研究了碳负载贵金属催化剂(Pt/C、Pd/C、Rh/C 和 Ru/C)上取代烯酮的催化脱氢。烯酮起始原料的脱氢和半氢化产生芳香族化合物(初级产品)、饱和环酮(次级产品)和环醇(次要产品)。 Pd/C 表现出最高的催化活性,其次是 Pt/C 和 Rh/C。即使有氢供体存在,芳香族化合物仍然是主要产物。联合实验和理论分析表明,四种催化材料可稳定金属表面上由酮-烯醇互变异构形成的常见二烯醇中间体。该中间体随后在脱氢时形成芳香族产物。烯酮反应物在催化表面上的结合方向强烈依赖于金属,因为 M-O 键距根据金属而显着变化。较长的 M-O 键(Pt:2.84 Å > Pd:2.23 Å > Rh:2.17 Å > Ru:2.07 Å)与更快的反应速率和更有利的酮-烯醇互变异构相关,因为较短的距离对应于更稳定的起始材料。互变异构化是通过逐步的表面辅助途径发生的。总体而言,每种研究的金属都表现出明显的活化焓和熵平衡(Δ H ° ‡ 、Δ S ° ‡ ),在烯酮脱氢反应领域提供了独特的可能性,这些反应可以通过适当选择催化材料来实现。
更新日期:2024-02-09
中文翻译:

碳载贵金属上烯酮脱氢和转移加氢生成酚和酮
在惰性条件下的有机相中研究了碳负载贵金属催化剂(Pt/C、Pd/C、Rh/C 和 Ru/C)上取代烯酮的催化脱氢。烯酮起始原料的脱氢和半氢化产生芳香族化合物(初级产品)、饱和环酮(次级产品)和环醇(次要产品)。 Pd/C 表现出最高的催化活性,其次是 Pt/C 和 Rh/C。即使有氢供体存在,芳香族化合物仍然是主要产物。联合实验和理论分析表明,四种催化材料可稳定金属表面上由酮-烯醇互变异构形成的常见二烯醇中间体。该中间体随后在脱氢时形成芳香族产物。烯酮反应物在催化表面上的结合方向强烈依赖于金属,因为 M-O 键距根据金属而显着变化。较长的 M-O 键(Pt:2.84 Å > Pd:2.23 Å > Rh:2.17 Å > Ru:2.07 Å)与更快的反应速率和更有利的酮-烯醇互变异构相关,因为较短的距离对应于更稳定的起始材料。互变异构化是通过逐步的表面辅助途径发生的。总体而言,每种研究的金属都表现出明显的活化焓和熵平衡(Δ H ° ‡ 、Δ S ° ‡ ),在烯酮脱氢反应领域提供了独特的可能性,这些反应可以通过适当选择催化材料来实现。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号