背景和目标
实验研究将功能失调的法尼醇 X 受体 (FXR)-成纤维细胞生长因子 19 (FGF19) 信号传导与肝脏疾病联系起来。本研究调查了 FXR-FGF19 通路沿肠肝轴的关键交叉点及其与肝硬化患者疾病严重程度的关系。
方法
接受肝静脉压力梯度测量的肝硬化患者(队列 I n = 107,包括n = 53 例同时进行肝活检; n = 5 名健康对照)或结肠镜检查并回肠活检(队列 II n = 37; n = 6 名对照)被包括在内。评估了反映 FXR 激活和肠道屏障完整性的肝脏和肠道基因表达。测量了全身胆汁酸 (BA) 和 FGF19 水平。
结果
全身 BA 和 FGF19 水平显着相关( r = 0.461; p < 0.001),并且随着肝硬化严重程度的增加而增加。肝硬化患者的肝脏 SHP 表达降低(与对照相比; p < 0.001),表明肝脏中 FXR 激活减少。全身 FGF19( r = -0.512, p < 0.001)和 BA( r = -0.487, p < 0.001)水平与肝脏 CYP7A1 表达呈负相关,但与 SHP 或 CYP8B1 表达无关,表明肝脏中的反馈信号受损。在回肠中,肝硬化患者中 FXR、SHP 和 FGF19 的表达降低,有趣的是,肠道 FGF19 表达与全身 FGF19 水平无关。回肠中肠闭锁小带-1、occludin 和 α-5-防御素的表达与 SHP 相关,并且与对照组相比,失代偿性肝硬化患者的表达降低。
结论
肝硬化患者肠肝轴的重要分子交叉点上的 FXR-FGF19 信号传导失调。回肠粘膜中 FXR 激活的减少与肠屏障蛋白表达的减少有关。这些人体数据需要针对肝硬化患者 FXR-FGF19 通路的干预措施进行进一步的机制研究。
临床试验号: NCT03267615
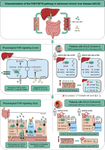
图文摘要
晚期慢性肝病 (ACLD) 患者肝肠 FXR-FGF19 信号传导的生理学及其调节。 (FXR)法尼醇X受体; (FGF19)成纤维细胞生长因子19; (BA)胆汁酸; (c/dACLD) 代偿性/失代偿性晚期慢性肝病; (FXR)法尼醇X受体; (SHP) 小异源二聚体伴侣; (OST-α/-β)有机溶质转运蛋白亚基α/β; (CYP7A1)胆固醇7α-羟化酶; (NTCP) Na+-牛磺胆酸盐共转运多肽; (CYP8B1)甾醇12-α-羟化酶; (HVPG)肝静脉压力梯度; (TJ) 紧密连接; (AMP) 抗菌肽; (ASBT) 顶端钠依赖性胆汁酸转运蛋白; (ZO 1) 闭合小带-1; (OCLN) 遮挡; (DEFA5) α-5-防御素。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
FXR-FGF19 signaling in the gut–liver axis is dysregulated in patients with cirrhosis and correlates with impaired intestinal defence
Background and aims
Experimental studies linked dysfunctional Farnesoid X receptor (FXR)-fibroblast growth factor 19 (FGF19) signaling to liver disease. This study investigated key intersections of the FXR-FGF19 pathway along the gut–liver axis and their link to disease severity in patients with cirrhosis.
Methods
Patients with cirrhosis undergoing hepatic venous pressure gradient measurement (cohort-I n = 107, including n = 53 with concomitant liver biopsy; n = 5 healthy controls) or colonoscopy with ileum biopsy (cohort-II n = 37; n = 6 controls) were included. Hepatic and intestinal gene expression reflecting FXR activation and intestinal barrier integrity was assessed. Systemic bile acid (BA) and FGF19 levels were measured.
Results
Systemic BA and FGF19 levels correlated significantly (r = 0.461; p < 0.001) and increased with cirrhosis severity. Hepatic SHP expression decreased in patients with cirrhosis (vs. controls; p < 0.001), indicating reduced FXR activation in the liver. Systemic FGF19 (r = −0.512, p < 0.001) and BA (r = −0.487, p < 0.001) levels correlated negatively with hepatic CYP7A1, but not SHP or CYP8B1 expression, suggesting impaired feedback signaling in the liver. In the ileum, expression of FXR, SHP and FGF19 decreased in patients with cirrhosis, and interestingly, intestinal FGF19 expression was not linked to systemic FGF19 levels. Intestinal zonula occludens-1, occludin, and alpha-5-defensin expression in the ileum correlated with SHP and decreased in patients with decompensated cirrhosis as compared to controls.
Conclusions
FXR-FGF19 signaling is dysregulated at essential molecular intersections along the gut–liver axis in patients with cirrhosis. Decreased FXR activation in the ileum mucosa was linked to reduced expression of intestinal barrier proteins. These human data call for further mechanistic research on interventions targeting the FXR-FGF19 pathway in patients with cirrhosis.
Clinical trial number: NCT03267615
Graphical abstract
Physiology of enterohepatic FXR-FGF19 signaling and its regulation in patients with advanced chronic liver disease (ACLD). (FXR) farnesoid X receptor; (FGF19) fibroblast growth factor 19; (BA) bile acids; (c/dACLD) compensated/decompensated advanced chronic liver disease; (FXR) farnesoid X receptor; (SHP) small heterodimer partner; (OST-α/-β) organic solute transporter subunit alpha/beta; (CYP7A1) cholesterol 7 alpha-hydroxylase; (NTCP) Na+-taurocholate cotransporting polypeptide; (CYP8B1) sterol 12-alpha-hydroxylase; (HVPG) hepatic venous pressure gradient; (TJ) tight junctions; (AMP) antimicrobial peptides; (ASBT) Apical Sodium Dependent Bile Acid Transporter; (ZO 1) zonula occludens-1; (OCLN) occluding; (DEFA5) alpha-5-defensin.


































 京公网安备 11010802027423号
京公网安备 11010802027423号