当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Transformations of 2-Nitro-1H-benzo[f]chromenes in Reactions with Alkylidenemalononitriles: Access to Naphtho[2,1-b]furans via Base-Mediated Pyran Ring Contraction
Organic Letters ( IF 4.9 ) Pub Date : 2024-02-08 , DOI: 10.1021/acs.orglett.3c03879
Kirill S Korzhenko 1 , Anastasiya S Yushkova 1 , Dmitry V Osipov 1 , Daria A Rashchepkina 1 , Oleg P Demidov 2 , Vitaly A Osyanin 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-02-08 , DOI: 10.1021/acs.orglett.3c03879
Kirill S Korzhenko 1 , Anastasiya S Yushkova 1 , Dmitry V Osipov 1 , Daria A Rashchepkina 1 , Oleg P Demidov 2 , Vitaly A Osyanin 1
Affiliation
|
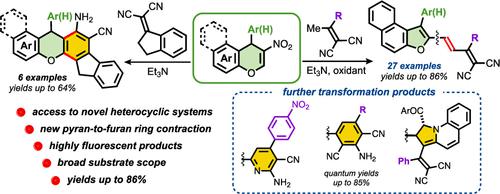
The action of 2-(1-arylethylidene)malononitriles on 2-nitro-1H-benzo[f]chromenes in the presence of Et3N and MoO3·2H2O results in naphtho[2,1-b]furans containing an allylidenemalononitrile unit in the α-position. The reaction proceeds with contraction of the pyran ring via a cascade carba-Michael addition/retro-oxa-Michael reaction/tautomerization/SN2/oxidation process. In contrast, the reaction of 2-nitro-1H-benzo[f]chromenes with the cyclic Knoevenagel adduct derived from 1-indanone and malononitrile leads to dihydroindeno[1,2-c]xanthenes. The possibility of further transformations of naphtho[2,1-b]furan derivatives as useful precursors and their optical properties were also investigated.
中文翻译:

2-硝基-1H-苯并[f]色烯与亚烷基丙二腈反应中的发散转化:通过碱介导的吡喃环收缩获得萘并[2,1-b]呋喃
在 Et 3 N 和 MoO 3 ·2H 2 O 存在下,2-(1-芳基亚乙基)丙二腈与 2-硝基-1 H-苯并[ f ]色烯反应生成含有以下成分的萘并[2,1 -b ]呋喃: α-位的烯丙二腈单元。该反应通过级联的carba-Michael加成/retro-oxa-Michael反应/互变异构/S N 2/氧化过程随着吡喃环的收缩而进行。相反,2-硝基-1H-苯并[ f ]色烯与衍生自1-茚满酮和丙二腈的环状Knoevenagel加合物的反应产生二氢茚并[1,2- c ]呫吨。还研究了萘并[2,1- b ]呋喃衍生物作为有用前体进一步转化的可能性及其光学性质。
更新日期:2024-02-08
中文翻译:

2-硝基-1H-苯并[f]色烯与亚烷基丙二腈反应中的发散转化:通过碱介导的吡喃环收缩获得萘并[2,1-b]呋喃
在 Et 3 N 和 MoO 3 ·2H 2 O 存在下,2-(1-芳基亚乙基)丙二腈与 2-硝基-1 H-苯并[ f ]色烯反应生成含有以下成分的萘并[2,1 -b ]呋喃: α-位的烯丙二腈单元。该反应通过级联的carba-Michael加成/retro-oxa-Michael反应/互变异构/S N 2/氧化过程随着吡喃环的收缩而进行。相反,2-硝基-1H-苯并[ f ]色烯与衍生自1-茚满酮和丙二腈的环状Knoevenagel加合物的反应产生二氢茚并[1,2- c ]呫吨。还研究了萘并[2,1- b ]呋喃衍生物作为有用前体进一步转化的可能性及其光学性质。































 京公网安备 11010802027423号
京公网安备 11010802027423号