当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Controllable Pyridine N-Oxidation–Nucleophilic Dechlorination Process for Enhanced Dechlorination of Chloropyridines: The Cooperation of HCO4– and HO2–
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-02-08 , DOI: 10.1021/acs.est.3c09878 Ying Chen 1, 2 , Lei Tian 1 , Wen Liu 3 , Yi Mei 1 , Qiu-Ju Xing 1 , Yi Mu 1 , Ling-Ling Zheng 1 , Qian Fu 1 , Jian-Ping Zou 1, 2 , Daishe Wu 2, 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-02-08 , DOI: 10.1021/acs.est.3c09878 Ying Chen 1, 2 , Lei Tian 1 , Wen Liu 3 , Yi Mei 1 , Qiu-Ju Xing 1 , Yi Mu 1 , Ling-Ling Zheng 1 , Qian Fu 1 , Jian-Ping Zou 1, 2 , Daishe Wu 2, 4
Affiliation
|
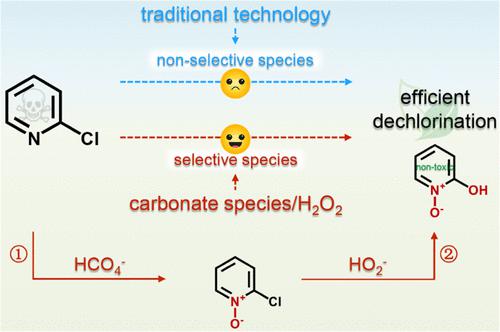
Dechlorination of chloropyridines can eliminate their detrimental environmental effects. However, traditional dechlorination technology cannot efficiently break the C–Cl bond of chloropyridines, which is restricted by the uncontrollable nonselective species. Hence, we propose the carbonate species-activated hydrogen peroxide (carbonate species/H2O2) process wherein the selective oxidant (peroxymonocarbonate ion, HCO4–) and selective reductant (hydroperoxide anion, HO2–) controllably coexist by manipulation of reaction pH. Taking 2-chloropyridine (Cl–Py) as an example, HCO4– first induces Cl–Py into pyridine N-oxidation intermediates, which then suffer from the nucleophilic dechlorination by HO2–. The obtained dechlorination efficiencies in the carbonate species/H2O2 process (32.5–84.5%) based on the cooperation of HCO4– and HO2– are significantly higher than those in the HO2–-mediated sodium hydroxide/hydrogen peroxide process (0–43.8%). Theoretical calculations confirm that pyridine N-oxidation of Cl–Py can effectively lower the energy barrier of the dechlorination process. Moreover, the carbonate species/H2O2 process exhibits superior anti-interference performance and low electric energy consumption. Furthermore, Cl–Py is completely detoxified via the carbonate species/H2O2 process. More importantly, the carbonate species/H2O2 process is applicable for efficient dehalogenation of halogenated pyridines and pyrazines. This work offers a simple and useful strategy to enhance the dehalogenation efficiency of halogenated organics and sheds new insights into the application of the carbonate species/H2O2 process in practical environmental remediation.
中文翻译:

可控吡啶 N-氧化-亲核脱氯过程增强氯吡啶脱氯:HCO4- 和 HO2- 的配合
氯吡啶的脱氯可以消除其对环境的有害影响。然而,传统的脱氯技术受到非选择性物种不可控的限制,无法有效破坏氯吡啶的C-Cl键。因此,我们提出了碳酸盐物种激活的过氧化氢(碳酸盐物种/H 2 O 2)工艺,其中选择性氧化剂(过一碳酸根离子,HCO 4 –)和选择性还原剂(氢过氧化物阴离子,HO 2 –)通过控制反应可控地共存酸碱度。以2-氯吡啶(Cl–Py)为例,HCO 4 –首先将Cl–Py诱导为吡啶N-氧化中间体,然后再被HO 2 –亲核脱氯。基于HCO 4 -和HO 2 -的配合,碳酸盐/H 2 O 2工艺获得的脱氯效率(32.5-84.5%)显着高于HO 2 -介导的氢氧化钠/过氧化氢工艺。 (0–43.8%)。理论计算证实,吡啶N-氧化Cl-Py可以有效降低脱氯过程的能垒。此外,碳酸盐/H 2 O 2工艺具有优异的抗干扰性能和较低的电能消耗。此外,Cl–Py 通过碳酸盐/H 2 O 2过程完全解毒。更重要的是,碳酸盐/H 2 O 2工艺适用于卤代吡啶和吡嗪的高效脱卤。这项工作提供了一种简单而有用的策略来提高卤代有机物的脱卤效率,并为碳酸盐/H 2 O 2过程在实际环境修复中的应用提供了新的见解。
更新日期:2024-02-08
中文翻译:

可控吡啶 N-氧化-亲核脱氯过程增强氯吡啶脱氯:HCO4- 和 HO2- 的配合
氯吡啶的脱氯可以消除其对环境的有害影响。然而,传统的脱氯技术受到非选择性物种不可控的限制,无法有效破坏氯吡啶的C-Cl键。因此,我们提出了碳酸盐物种激活的过氧化氢(碳酸盐物种/H 2 O 2)工艺,其中选择性氧化剂(过一碳酸根离子,HCO 4 –)和选择性还原剂(氢过氧化物阴离子,HO 2 –)通过控制反应可控地共存酸碱度。以2-氯吡啶(Cl–Py)为例,HCO 4 –首先将Cl–Py诱导为吡啶N-氧化中间体,然后再被HO 2 –亲核脱氯。基于HCO 4 -和HO 2 -的配合,碳酸盐/H 2 O 2工艺获得的脱氯效率(32.5-84.5%)显着高于HO 2 -介导的氢氧化钠/过氧化氢工艺。 (0–43.8%)。理论计算证实,吡啶N-氧化Cl-Py可以有效降低脱氯过程的能垒。此外,碳酸盐/H 2 O 2工艺具有优异的抗干扰性能和较低的电能消耗。此外,Cl–Py 通过碳酸盐/H 2 O 2过程完全解毒。更重要的是,碳酸盐/H 2 O 2工艺适用于卤代吡啶和吡嗪的高效脱卤。这项工作提供了一种简单而有用的策略来提高卤代有机物的脱卤效率,并为碳酸盐/H 2 O 2过程在实际环境修复中的应用提供了新的见解。








































 京公网安备 11010802027423号
京公网安备 11010802027423号