当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Nidulalin A and Nidulaxanthone A: Selective Carbonyl Desaturation Using an Oxoammonium Salt
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-02-06 , DOI: 10.1021/jacs.3c13864 Kaijie Ji 1, 2 , Richard P Johnson 3 , James McNeely 2 , Md Al Faruk 3 , John A Porco 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-02-06 , DOI: 10.1021/jacs.3c13864 Kaijie Ji 1, 2 , Richard P Johnson 3 , James McNeely 2 , Md Al Faruk 3 , John A Porco 1, 2
Affiliation
|
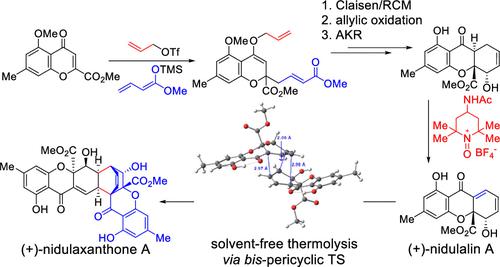
Nidulaxanthone A is a dimeric, dihydroxanthone natural product that was isolated in 2020 from Aspergillus sp. Structurally, the compound features an unprecedented heptacyclic 6/6/6/6/6/6/6 ring system which is unusual for natural xanthone dimers. Biosynthetically, nidulaxanthone A originates from the monomer nidulalin A via stereoselective Diels–Alder dimerization. To expedite the synthesis of nidulalin A and study the proposed dimerization, we developed methodology involving the use of allyl triflate for chromone ester activation, followed by vinylogous addition, to rapidly forge the nidulalin A scaffold in a four-step sequence which also features ketone desaturation using Bobbitt’s oxoammonium salt. An asymmetric synthesis of nidulalin A was achieved using acylative kinetic resolution (AKR) of chiral, racemic 2H-nidulalin A. Dimerization of enantioenriched nidulalin A to nidulaxanthone A was achieved using solvent-free, thermolytic conditions. Computational studies have been conducted to probe both the oxoammonium-mediated desaturation and (4 + 2) dimerization events.
中文翻译:

Nidulalin A 和 Nidulaxanthone A 的不对称合成:使用氧铵盐选择性羰基去饱和
Nidulaxanthone A 是一种二聚二氢氧杂蒽酮天然产物,于 2020 年从曲霉属 sp中分离出来。在结构上,该化合物具有前所未有的七环 6/6/6/6/6/6/6 环系统,这对于天然呫吨酮二聚体来说是不常见的。在生物合成上,nidulaxanthone A 源自单体 nidulalin A 通过立体选择性 Diels-Alder 二聚化。为了加速 Nidulalin A 的合成并研究所提出的二聚化,我们开发了一种方法,包括使用三氟甲磺酸烯丙酯进行色酮酯活化,然后进行插烯加成,以四步顺序快速锻造 Nidulalin A 支架,该支架还具有酮去饱和作用使用博比特的氧铵盐。使用手性外消旋 2 H -nidulalin A 的酰化动力学拆分 (AKR) 实现了 nidulalin A 的不对称合成。使用无溶剂热解条件实现了对映体富集的 nidulalin A 向 nidulaxanthone A 的二聚化。已进行计算研究来探测氧铵介导的去饱和和 (4 + 2) 二聚化事件。
更新日期:2024-02-06
中文翻译:

Nidulalin A 和 Nidulaxanthone A 的不对称合成:使用氧铵盐选择性羰基去饱和
Nidulaxanthone A 是一种二聚二氢氧杂蒽酮天然产物,于 2020 年从曲霉属 sp中分离出来。在结构上,该化合物具有前所未有的七环 6/6/6/6/6/6/6 环系统,这对于天然呫吨酮二聚体来说是不常见的。在生物合成上,nidulaxanthone A 源自单体 nidulalin A 通过立体选择性 Diels-Alder 二聚化。为了加速 Nidulalin A 的合成并研究所提出的二聚化,我们开发了一种方法,包括使用三氟甲磺酸烯丙酯进行色酮酯活化,然后进行插烯加成,以四步顺序快速锻造 Nidulalin A 支架,该支架还具有酮去饱和作用使用博比特的氧铵盐。使用手性外消旋 2 H -nidulalin A 的酰化动力学拆分 (AKR) 实现了 nidulalin A 的不对称合成。使用无溶剂热解条件实现了对映体富集的 nidulalin A 向 nidulaxanthone A 的二聚化。已进行计算研究来探测氧铵介导的去饱和和 (4 + 2) 二聚化事件。






























 京公网安备 11010802027423号
京公网安备 11010802027423号