当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosted Light Alkane Deep Oxidation via Metal Bond Length Modulation-Induced C–C Bond Preferential Activation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-02-06 , DOI: 10.1021/acs.est.3c06916 Lianghui Xia 1 , Yanfei Jian 1 , Qiyuan Liu 1 , Yujie Liu 1 , Jingjing Wang 1 , Shouning Chai 1 , Meizan Jing 2 , Reem Albilali 3 , Chi He 1, 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-02-06 , DOI: 10.1021/acs.est.3c06916 Lianghui Xia 1 , Yanfei Jian 1 , Qiyuan Liu 1 , Yujie Liu 1 , Jingjing Wang 1 , Shouning Chai 1 , Meizan Jing 2 , Reem Albilali 3 , Chi He 1, 4
Affiliation
|
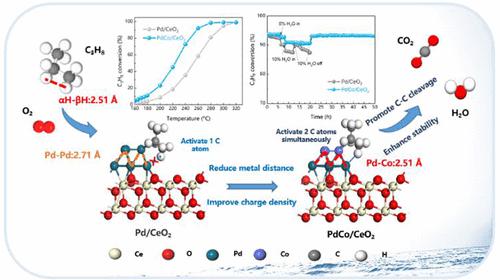
Light alkanes (LAs), typical VOCs existing in both stationary and mobile sources, pose significant environmental concerns. Although noble metal catalysts demonstrate strong C–H bond activation, their effectiveness in degrading LAs is hindered by inherent challenges, including poor chemical stability and water resistance. Here, from a new perspective, we propose a feasible strategy that adjusting the metal bond lengths within Pd clusters through partial substitution of smaller radius 3d transition metals (3dTMs) to prioritize the activation of low-energy C–C bonds within LAs. Benefiting from this, PdCo/CeO2 exhibits exceptional catalytic performance in propane degradation due to their high capacity for C–C cleavage stemming from the shorter Pd–Co length (2.51 Å) and lower coordination number (1.73), boosting the activation of α-H and β-H of propane simultaneously and accelerating the mobility of postactivated oxygen species to prevent Pd center deep oxidation. The presence of 3dTMs on Pd clusters improves the redox and charge transfer ability of catalysts, resulting in an amplified generation of oxygen vacancies and facilitating the adsorption and activation of reactants. Mechanistic studies and DFT calculations suggest that the substitution of 3dTMs significantly accelerate C–C bond cleavage within C3 intermediates to generate the subsequent C2 and C1 intermediates, suppressing the generation of harmful byproducts.
中文翻译:

通过金属键长调节诱导 C-C 键优先激活促进轻质烷烃深度氧化
轻烷烃 (LA) 是固定源和移动源中存在的典型挥发性有机化合物,会造成严重的环境问题。尽管贵金属催化剂表现出很强的 C-H 键活化作用,但其降解 LA 的有效性受到固有挑战的阻碍,包括化学稳定性差和耐水性差。在这里,我们从一个新的角度提出了一种可行的策略,即通过部分取代较小半径的3d过渡金属(3dTM)来调整Pd簇内的金属键长,以优先激活LA内的低能C-C键。受益于此,PdCo/CeO 2在丙烷降解中表现出优异的催化性能,因为较短的 Pd-Co 长度 (2.51 Å) 和较低的配位数 (1.73) 导致其具有较高的 C-C 裂解能力,从而促进了 α 的活化同时抑制丙烷的-H和β-H,加速后活化氧的迁移,防止Pd中心深度氧化。 Pd簇上3dTM的存在提高了催化剂的氧化还原和电荷转移能力,导致氧空位的产生放大,促进反应物的吸附和活化。机理研究和DFT计算表明,3dTM的取代显着加速了C3中间体中的C-C键断裂,生成后续的C2和C1中间体,抑制有害副产物的产生。
更新日期:2024-02-06
中文翻译:

通过金属键长调节诱导 C-C 键优先激活促进轻质烷烃深度氧化
轻烷烃 (LA) 是固定源和移动源中存在的典型挥发性有机化合物,会造成严重的环境问题。尽管贵金属催化剂表现出很强的 C-H 键活化作用,但其降解 LA 的有效性受到固有挑战的阻碍,包括化学稳定性差和耐水性差。在这里,我们从一个新的角度提出了一种可行的策略,即通过部分取代较小半径的3d过渡金属(3dTM)来调整Pd簇内的金属键长,以优先激活LA内的低能C-C键。受益于此,PdCo/CeO 2在丙烷降解中表现出优异的催化性能,因为较短的 Pd-Co 长度 (2.51 Å) 和较低的配位数 (1.73) 导致其具有较高的 C-C 裂解能力,从而促进了 α 的活化同时抑制丙烷的-H和β-H,加速后活化氧的迁移,防止Pd中心深度氧化。 Pd簇上3dTM的存在提高了催化剂的氧化还原和电荷转移能力,导致氧空位的产生放大,促进反应物的吸附和活化。机理研究和DFT计算表明,3dTM的取代显着加速了C3中间体中的C-C键断裂,生成后续的C2和C1中间体,抑制有害副产物的产生。

































 京公网安备 11010802027423号
京公网安备 11010802027423号