当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor Microenvironment ROS/pH Cascade-Responsive Supramolecular Nanoplatform with ROS Regeneration Property for Enhanced Hepatocellular Carcinoma Therapy
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-02-05 , DOI: 10.1021/acsami.3c16022 Jinfeng Shi 1, 2 , Yehui Wang 3 , Yihan Wu 1 , Jingjing Li 4 , Chaomei Fu 1 , Yi Li 2 , Xingliang Xie 2 , Xiaohong Fan 3 , Yichen Hu 5 , Chuan Hu 1 , Jinming Zhang 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2024-02-05 , DOI: 10.1021/acsami.3c16022 Jinfeng Shi 1, 2 , Yehui Wang 3 , Yihan Wu 1 , Jingjing Li 4 , Chaomei Fu 1 , Yi Li 2 , Xingliang Xie 2 , Xiaohong Fan 3 , Yichen Hu 5 , Chuan Hu 1 , Jinming Zhang 1
Affiliation
|
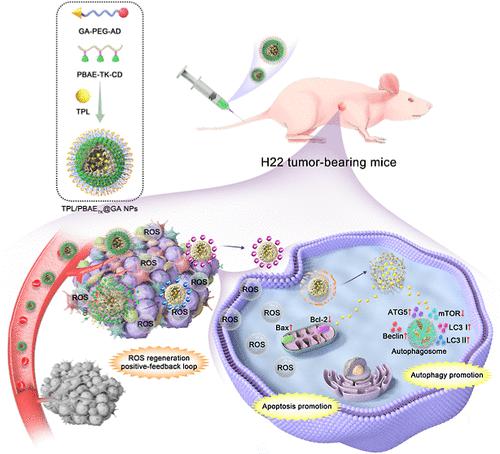
The low targeted drug delivery efficiency, including poor tumor accumulation and penetration and uncontrolled drug release, leads to the failure of cancer therapy. Herein, a multifunctional supramolecular nanoplatform loading triptolide (TPL/PBAETK@GA NPs) was fabricated via the host–guest interaction between glycyrrhetinic-acid-modified poly(ethylene glycol)-adamantanecarboxylic acid moiety and reactive oxygen species (ROS)/pH cascade-responsive copolymer poly(β-amino esters)-thioketal (TK)-β-cyclodextrin. TPL/PBAETK@GA NPs could accumulate in hepatocellular carcinoma (HCC) tissue effectively, mediated by nanoscale advantage and GA’ recognition to specific receptors. The elevated concentration of ROS in tumor microenvironment (TME) quickly breaks the TK linkages, causing the detachment of shell (cyclodextrin) CD layer. Then, the accompanying negative-to-positive charge-reversal of NPs was realized via the PBAE moiety protonation under the slightly acidic TME, significantly enhancing the NPs’ cellular internalization. Remarkably, the pH-responsive endo/lysosome escape of PBAE core triggered intracellular TPL burst release, promoting the cancer cell apoptosis, autophagy, and intracellular ROS generation, leading to the self-amplification of ROS in TME. Afterward, the ROS positive-feedback loop was generated to further promote size-shrinkage and charge-reversal of NPs. Both in vitro and in vivo tests verified that TPL/PBAETK@GA NPs produced a satisfactory anti-HCC therapy outcome. Collectively, this study offers a potential appealing paradigm to enhance TPL-based HCC therapy outcomes via multifunctionalized supramolecular nanodrugs.
中文翻译:

具有 ROS 再生特性的肿瘤微环境 ROS/pH 级联响应超分子纳米平台,用于增强肝细胞癌治疗
靶向药物递送效率低,包括肿瘤积聚和渗透不良以及药物释放失控,导致癌症治疗失败。在此,通过甘草次酸修饰的聚(乙二醇)-金刚烷甲酸部分和活性氧(ROS)/pH级联之间的主客体相互作用,制备了负载雷公藤内酯醇的多功能超分子纳米平台(TPL/PBAE TK @GA NPs) -响应性共聚物聚(β-氨基酯)-缩硫酮(TK)-β-环糊精。通过纳米级优势和 GA 对特定受体的识别介导,TPL/PBAE TK @GA NPs 可以在肝细胞癌 (HCC) 组织中有效积累。肿瘤微环境(TME)中ROS浓度升高迅速破坏TK连接,导致壳(环糊精)CD层脱离。然后,在微酸性 TME 下通过 PBAE 部分质子化实现了 NP 的负电荷反转,显着增强了 NP 的细胞内化。值得注意的是,PBAE 核心的 pH 响应性内切/溶酶体逃逸触发了细胞内 TPL 爆发释放,促进癌细胞凋亡、自噬和细胞内 ROS 生成,导致 TME 中 ROS 的自我扩增。随后,产生ROS正反馈环路,进一步促进纳米粒子的尺寸收缩和电荷反转。体外和体内测试均证实 TPL/PBAE TK @GA NPs 产生了令人满意的抗 HCC 治疗结果。总的来说,这项研究提供了一个潜在的有吸引力的范例,通过多功能超分子纳米药物来增强基于 TPL 的 HCC 治疗效果。
更新日期:2024-02-05
中文翻译:

具有 ROS 再生特性的肿瘤微环境 ROS/pH 级联响应超分子纳米平台,用于增强肝细胞癌治疗
靶向药物递送效率低,包括肿瘤积聚和渗透不良以及药物释放失控,导致癌症治疗失败。在此,通过甘草次酸修饰的聚(乙二醇)-金刚烷甲酸部分和活性氧(ROS)/pH级联之间的主客体相互作用,制备了负载雷公藤内酯醇的多功能超分子纳米平台(TPL/PBAE TK @GA NPs) -响应性共聚物聚(β-氨基酯)-缩硫酮(TK)-β-环糊精。通过纳米级优势和 GA 对特定受体的识别介导,TPL/PBAE TK @GA NPs 可以在肝细胞癌 (HCC) 组织中有效积累。肿瘤微环境(TME)中ROS浓度升高迅速破坏TK连接,导致壳(环糊精)CD层脱离。然后,在微酸性 TME 下通过 PBAE 部分质子化实现了 NP 的负电荷反转,显着增强了 NP 的细胞内化。值得注意的是,PBAE 核心的 pH 响应性内切/溶酶体逃逸触发了细胞内 TPL 爆发释放,促进癌细胞凋亡、自噬和细胞内 ROS 生成,导致 TME 中 ROS 的自我扩增。随后,产生ROS正反馈环路,进一步促进纳米粒子的尺寸收缩和电荷反转。体外和体内测试均证实 TPL/PBAE TK @GA NPs 产生了令人满意的抗 HCC 治疗结果。总的来说,这项研究提供了一个潜在的有吸引力的范例,通过多功能超分子纳米药物来增强基于 TPL 的 HCC 治疗效果。































 京公网安备 11010802027423号
京公网安备 11010802027423号