当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design and synthesis of novel chloropyridazine hybrids as promising anticancer agents acting by apoptosis induction and PARP-1 inhibition through a molecular hybridization strategy
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2024-02-06 , DOI: 10.1039/d3md00751k Norhan A. Abdelrahman 1 , Ahmed A. Al-Karmalawy 2, 3 , Maiy Y. Jaballah 1 , Galal Yahya 4, 5 , Marwa Sharaky 6, 7 , Khaled A. M. Abouzid 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2024-02-06 , DOI: 10.1039/d3md00751k Norhan A. Abdelrahman 1 , Ahmed A. Al-Karmalawy 2, 3 , Maiy Y. Jaballah 1 , Galal Yahya 4, 5 , Marwa Sharaky 6, 7 , Khaled A. M. Abouzid 1
Affiliation
|
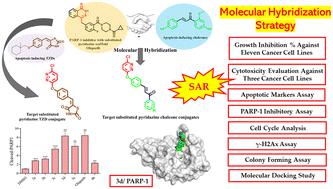
Guided by the molecular hybridization principle, a novel series of 4-chloropyridazinoxyphenyl conjugates (3a–h, 4a–e, and 5) was designed and synthesized as proposed apoptotic inducers and PARP-1 inhibitors. The growth inhibition % of the designed hybrids was investigated in eleven cancer cell lines, where the anticancer activities were found to be in the following order: 4-chloropyridazinoxyphenyl-aromatic ketones hybrids (3a–h) > 4-chloropyridazinoxyphenyl-benzyloxyphenylethan-1-one hybrids (4a–e) > 4-chloropyridazinoxyphenyl-thiazolidine-2,4-dione hybrid (5). Further, the most sensitive three cancer cell lines (HNO97, FaDu, and MDA-MB-468) were selected to measure the IC50 values of the new hybrids. Moreover, the frontier three members (3c, 3e, and 4b) were selected for the measurements of apoptotic protein markers (p53, BAX, caspase 3, caspase 6, BCL-2, and CK 18). Besides, the impact of compounds 3a–e and 4b on the activity of PARP-1 was investigated, where 3c, 3d, and 3e demonstrated comparable efficiencies to olaparib. Furthermore, γ-H2Ax, a well-established marker for double-strand DNA breaks, was examined and the occurrence of DNA damage was observed. In addition, a significant inhibition of cell proliferation and a remarkable 15 to 50-fold reduction in the number of colonies compared to the control group were recorded. Finally, the PARP-1 inhibitory potential of the novel hybrids was compared to the co-crystal of the target receptor (PDB ID: 6NTU) using molecular docking.
中文翻译:

设计和合成新型氯哒嗪杂合体作为有前景的抗癌药物,通过分子杂交策略诱导细胞凋亡和抑制 PARP-1
在分子杂交原理的指导下,设计并合成了一系列新型4-氯哒嗪氧基苯基缀合物(3a-h、4a-e和5 )作为细胞凋亡诱导剂和PARP-1抑制剂。在 11 种癌细胞系中研究了设计的杂合体的生长抑制百分比,发现其抗癌活性按以下顺序排列: 4-氯哒嗪氧基苯基-芳香酮杂合体 ( 3a–h ) > 4-氯哒嗪氧基苯基-苄氧基苯基乙烷-1-一种杂化物 ( 4a–e ) > 4-氯哒嗪氧基苯基-噻唑烷-2,4-二酮杂化物 ( 5 )。此外,选择最敏感的三种癌细胞系(HNO97、FaDu和MDA-MB-468)来测量新杂交体的IC 50值。此外,选择前沿三个成员(3c、3e和4b)来测量凋亡蛋白标记物(p53、BAX、caspase 3、caspase 6、BCL-2和CK 18)。此外,还研究了化合物3a-e和4b对 PARP-1 活性的影响,其中3c、3d和3e表现出与奥拉帕尼相当的效率。此外,还检查了 γ-H2Ax(一种成熟的双链 DNA 断裂标记)并观察了 DNA 损伤的发生。此外,与对照组相比,细胞增殖受到显着抑制,集落数量显着减少 15 至 50 倍。最后,使用分子对接将新型杂交体的 PARP-1 抑制潜力与目标受体的共晶 (PDB ID:6NTU) 进行比较。
更新日期:2024-02-06
中文翻译:

设计和合成新型氯哒嗪杂合体作为有前景的抗癌药物,通过分子杂交策略诱导细胞凋亡和抑制 PARP-1
在分子杂交原理的指导下,设计并合成了一系列新型4-氯哒嗪氧基苯基缀合物(3a-h、4a-e和5 )作为细胞凋亡诱导剂和PARP-1抑制剂。在 11 种癌细胞系中研究了设计的杂合体的生长抑制百分比,发现其抗癌活性按以下顺序排列: 4-氯哒嗪氧基苯基-芳香酮杂合体 ( 3a–h ) > 4-氯哒嗪氧基苯基-苄氧基苯基乙烷-1-一种杂化物 ( 4a–e ) > 4-氯哒嗪氧基苯基-噻唑烷-2,4-二酮杂化物 ( 5 )。此外,选择最敏感的三种癌细胞系(HNO97、FaDu和MDA-MB-468)来测量新杂交体的IC 50值。此外,选择前沿三个成员(3c、3e和4b)来测量凋亡蛋白标记物(p53、BAX、caspase 3、caspase 6、BCL-2和CK 18)。此外,还研究了化合物3a-e和4b对 PARP-1 活性的影响,其中3c、3d和3e表现出与奥拉帕尼相当的效率。此外,还检查了 γ-H2Ax(一种成熟的双链 DNA 断裂标记)并观察了 DNA 损伤的发生。此外,与对照组相比,细胞增殖受到显着抑制,集落数量显着减少 15 至 50 倍。最后,使用分子对接将新型杂交体的 PARP-1 抑制潜力与目标受体的共晶 (PDB ID:6NTU) 进行比较。











































 京公网安备 11010802027423号
京公网安备 11010802027423号