当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Diagram Analysis of High-Pressure/High-Temperature Polymorphs of Ammonia Borane
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-02-05 , DOI: 10.1021/acs.inorgchem.3c03615 Satoshi Nakano 1 , Hiroshi Fujihisa 2 , Hiroshi Yamawaki 2 , Takumi Kikegawa 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2024-02-05 , DOI: 10.1021/acs.inorgchem.3c03615 Satoshi Nakano 1 , Hiroshi Fujihisa 2 , Hiroshi Yamawaki 2 , Takumi Kikegawa 3
Affiliation
|
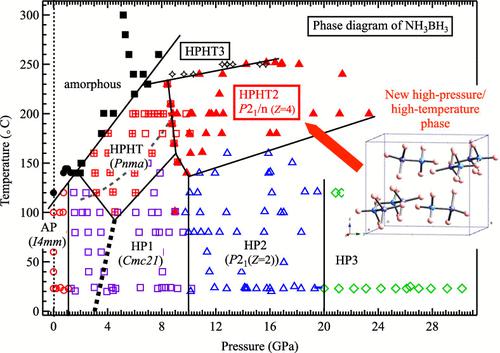
Ammonia borane (NH3BH3) is a promising hydrogen-storage material because of its high hydrogen density. It is employed as a hydrogen source when synthesizing superconducting polyhydrides under high pressure. Additionally, NH3BH3 is a crystallographically interesting compound that features protonic hydrogen (Hδ+) and hydridic hydrogen (Hδ−), and it forms a dihydrogen bond, which explains its stable existence as a solid. Herein, X-ray diffraction experiments were performed at high pressures (HPs) and high temperatures (HTs) of up to 30 GPa and 300 °C, respectively, to investigate the HP/HT phase diagram of NH3BH3. A new HP/HT phase (HPHT2) was identified above 9 GPa and 150 °C. Crystal-structure analysis using the Rietveld method and stability verification using density functional theory calculations revealed that HPHT2 has a P21/n (Z = 4) structure, similar to that of a previously reported HP/HT phase (HPHT) that appears at a lower pressure. HPHT2 is denser than the HP phases that appear at room temperature (HP1 and HP2) at the same pressure (up to ∼17 GPa). In the phase diagram, the phase-boundary line between HPHT and HP1 is a downward convex curve. These unconventional phenomena in the density and phase boundary can be attributed to the influence of dihydrogen bonding on the crystal structure and phase diagram.
中文翻译:

氨硼烷高压/高温多晶型物的相图分析
氨硼烷(NH 3 BH 3 )由于其高氢密度而成为一种很有前途的储氢材料。它在高压下合成超导聚氢化物时用作氢源。此外,NH 3 BH 3是一种晶体学上有趣的化合物,具有质子氢 (H δ+ ) 和氢氢 (H δ− ),并形成二氢键,这解释了它作为固体稳定存在。在此,分别在高达30 GPa和300℃的高压(HP)和高温(HT)下进行X射线衍射实验,以研究NH 3 BH 3的HP/HT相图。在 9 GPa 和 150 °C 以上发现了新的 HP/HT 相 (HPHT2)。使用 Rietveld 方法的晶体结构分析和使用密度泛函理论计算的稳定性验证表明,HPHT2 具有P 2 1 / n ( Z = 4) 结构,类似于之前报道的 HP/HT 相 (HPHT),该结构出现在较低的压力。 HPHT2 比室温下相同压力(高达~17 GPa)下出现的 HP 相(HP1 和 HP2)更致密。在相图中,HPHT和HP1之间的相界线是向下凸的曲线。这些密度和相界的非常规现象可归因于二氢键对晶体结构和相图的影响。
更新日期:2024-02-05
中文翻译:

氨硼烷高压/高温多晶型物的相图分析
氨硼烷(NH 3 BH 3 )由于其高氢密度而成为一种很有前途的储氢材料。它在高压下合成超导聚氢化物时用作氢源。此外,NH 3 BH 3是一种晶体学上有趣的化合物,具有质子氢 (H δ+ ) 和氢氢 (H δ− ),并形成二氢键,这解释了它作为固体稳定存在。在此,分别在高达30 GPa和300℃的高压(HP)和高温(HT)下进行X射线衍射实验,以研究NH 3 BH 3的HP/HT相图。在 9 GPa 和 150 °C 以上发现了新的 HP/HT 相 (HPHT2)。使用 Rietveld 方法的晶体结构分析和使用密度泛函理论计算的稳定性验证表明,HPHT2 具有P 2 1 / n ( Z = 4) 结构,类似于之前报道的 HP/HT 相 (HPHT),该结构出现在较低的压力。 HPHT2 比室温下相同压力(高达~17 GPa)下出现的 HP 相(HP1 和 HP2)更致密。在相图中,HPHT和HP1之间的相界线是向下凸的曲线。这些密度和相界的非常规现象可归因于二氢键对晶体结构和相图的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号