当前位置:
X-MOL 学术
›
J. Ethnopharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chaihu Guizhi Ganjiang Decoction attenuates nonalcoholic steatohepatitis by enhancing intestinal barrier integrity and ameliorating PPARα mediated lipotoxicity
Journal of Ethnopharmacology ( IF 4.8 ) Pub Date : 2024-02-03 , DOI: 10.1016/j.jep.2024.117841 Hao Wu 1 , Tianyu Lou 1 , Mingxia Pan 1 , Zuying Wei 1 , Xiaoqin Yang 1 , Lirong Liu 1 , Menghan Feng 1 , Lixia Shi 1 , Biqiong Qu 1 , Shiyu Cong 1 , Kui Chen 1 , Haolan Yang 1 , Jie Liu 2 , Yueting Li 1 , Zhixin Jia 2 , Hongbin Xiao 3
Journal of Ethnopharmacology ( IF 4.8 ) Pub Date : 2024-02-03 , DOI: 10.1016/j.jep.2024.117841 Hao Wu 1 , Tianyu Lou 1 , Mingxia Pan 1 , Zuying Wei 1 , Xiaoqin Yang 1 , Lirong Liu 1 , Menghan Feng 1 , Lixia Shi 1 , Biqiong Qu 1 , Shiyu Cong 1 , Kui Chen 1 , Haolan Yang 1 , Jie Liu 2 , Yueting Li 1 , Zhixin Jia 2 , Hongbin Xiao 3
Affiliation
|
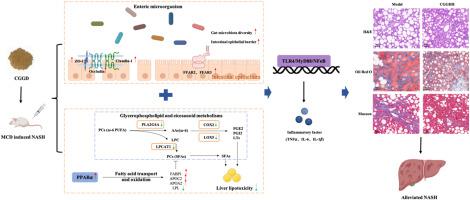
Nonalcoholic steatohepatitis (NASH) is a prominent cause of liver-related death that poses a threat to global health and is characterized by severe hepatic steatosis, lobular inflammation, and ballooning degeneration. To date, no Food and Drug Administration-approved medicine is commercially available. The Chaihu Guizhi Ganjiang Decoction (CGGD) shows potential curative effects on regulation of blood lipids and blood glucose, mitigation of organism inflammation, and amelioration of hepatic function. However, the overall regulatory mechanisms underlying its effects on NASH remain unclear. This study aimed to investigate the efficiency of CGGD on methionine- and choline-deficient (MCD)-induced NASH and unravel its underlying mechanisms. A NASH model of SD rats was established using an MCD diet for 8 weeks, and the efficacy of CGGD was evaluated based on hepatic lipid accumulation, inflammatory response, and fibrosis. The effects of CGGD on the intestinal barrier, metabolic profile, and differentially expressed genes (DEGs) profile were analyzed by integrating gut microbiota, metabolomics, and transcriptome sequencing to elucidate its mechanisms of action. In MCD-induced NASH rats, pathological staining demonstrated that CGGD alleviated lipid accumulation, inflammatory cell infiltration, and fibrosis in the hepatic tissue. After CGGD administration, liver index, liver weight, serum alanine aminotransferase (ALT), and aspartate aminotransferase (AST) contents, liver triglycerides (TG), and free fatty acids (FFAs) were decreased, meanwhile, it down-regulated the level of proinflammatory mediators (TNF-α, IL-6, IL-1β, MCP-1), and up-regulated the level of anti-inflammatory factors (IL-4, IL-10), and the expression of liver fibrosis markers TGFβ, Acta2, Col1a1 and Col1a2 were weakened. Mechanistically, CGGD treatment altered the diversity of intestinal flora, as evidenced by the depletion of , , , and enrichment of the probiotic genera , , , etc. The colonic histopathological results indicated that the gut barrier damage recovered in the CGGD treatment group, and the expression levels of colonic short-chain fatty acids (SCFAs)-specific receptors FFAR2, FFAR3, and tight junction (TJs) proteins ZO-1, Occludin, Claudin-1 were increased compared with those in the model group. Further metabolomic and transcriptomic analyses suggested that CGGD mitigated the lipotoxicity caused by glycerophospholipid and eicosanoid metabolism disorders by decreasing the levels of PLA2G4A, LPCAT1, COX2, and LOX5. In addition, CGGD could activate the inhibitory lipotoxic transcription factor PPARα, regulate the proteins of FABP1, APOC2, APOA2, and LPL to promote fatty acid catabolism, and suppress the TLR4/MyD88/NFκB pathway to attenuate NASH. Our study demonstrated that CGGD improved steatosis, inflammation, and fibrosis on NASH through enhancing intestinal barrier integrity and alleviating PPARα mediated lipotoxicity, which makes it an attractive candidate for potential new strategies for NASH prevention and treatment.
中文翻译:

柴胡桂枝干姜汤通过增强肠道屏障完整性和改善 PPARα 介导的脂毒性来减轻非酒精性脂肪性肝炎
非酒精性脂肪性肝炎 (NASH) 是肝脏相关死亡的主要原因,对全球健康构成威胁,其特点是严重的肝脂肪变性、小叶炎症和气球样变性。迄今为止,还没有经过食品和药物管理局批准的药物在市场上销售。柴胡桂枝干姜汤(CGGD)在调节血脂和血糖、减轻机体炎症和改善肝功能方面具有潜在的疗效。然而,其对 NASH 影响的整体调控机制仍不清楚。本研究旨在探讨 CGGD 对蛋氨酸和胆碱缺乏 (MCD) 诱导的 NASH 的功效,并揭示其潜在机制。采用MCD饮食建立SD大鼠NASH模型8周,根据肝脏脂质积累、炎症反应和纤维化情况评价CGGD的疗效。通过整合肠道微生物群、代谢组学和转录组测序,分析 CGGD 对肠道屏障、代谢谱和差异表达基因 (DEG) 谱的影响,以阐明其作用机制。在 MCD 诱导的 NASH 大鼠中,病理染色表明 CGGD 减轻了肝组织中的脂质积累、炎症细胞浸润和纤维化。 CGGD给药后,肝脏指数、肝脏重量、血清丙氨酸转氨酶(ALT)、天门冬氨酸转氨酶(AST)含量、肝脏甘油三酯(TG)、游离脂肪酸(FFA)下降,同时下调促炎介质(TNF-α、IL-6、IL-1β、MCP-1),上调抗炎因子(IL-4、IL-10)水平以及肝纤维化标志物 TGFβ 的表达, Acta2、Col1a1 和 Col1a2 被削弱。从机制上讲,CGGD 治疗改变了肠道菌群的多样性,如 、 、 、 和益生菌属的富集等所证明。结肠组织病理学结果表明 CGGD 治疗组的肠道屏障损伤得到恢复,并且与模型组相比,结肠短链脂肪酸(SCFA)特异性受体FFAR2、FFAR3和紧密连接(TJ)蛋白ZO-1、Occludin、Claudin-1的表达水平升高。进一步的代谢组学和转录组学分析表明,CGGD 通过降低 PLA2G4A、LPCAT1、COX2 和 LOX5 的水平来减轻甘油磷脂和类二十烷酸代谢紊乱引起的脂毒性。此外,CGGD还可以激活抑制性脂毒性转录因子PPARα,调节FABP1、APOC2、APOA2和LPL蛋白以促进脂肪酸分解代谢,并抑制TLR4/MyD88/NFκB通路以减轻NASH。我们的研究表明,CGGD 通过增强肠道屏障完整性和减轻 PPARα 介导的脂毒性来改善 NASH 的脂肪变性、炎症和纤维化,这使其成为 NASH 预防和治疗潜在新策略的有吸引力的候选者。
更新日期:2024-02-03
中文翻译:

柴胡桂枝干姜汤通过增强肠道屏障完整性和改善 PPARα 介导的脂毒性来减轻非酒精性脂肪性肝炎
非酒精性脂肪性肝炎 (NASH) 是肝脏相关死亡的主要原因,对全球健康构成威胁,其特点是严重的肝脂肪变性、小叶炎症和气球样变性。迄今为止,还没有经过食品和药物管理局批准的药物在市场上销售。柴胡桂枝干姜汤(CGGD)在调节血脂和血糖、减轻机体炎症和改善肝功能方面具有潜在的疗效。然而,其对 NASH 影响的整体调控机制仍不清楚。本研究旨在探讨 CGGD 对蛋氨酸和胆碱缺乏 (MCD) 诱导的 NASH 的功效,并揭示其潜在机制。采用MCD饮食建立SD大鼠NASH模型8周,根据肝脏脂质积累、炎症反应和纤维化情况评价CGGD的疗效。通过整合肠道微生物群、代谢组学和转录组测序,分析 CGGD 对肠道屏障、代谢谱和差异表达基因 (DEG) 谱的影响,以阐明其作用机制。在 MCD 诱导的 NASH 大鼠中,病理染色表明 CGGD 减轻了肝组织中的脂质积累、炎症细胞浸润和纤维化。 CGGD给药后,肝脏指数、肝脏重量、血清丙氨酸转氨酶(ALT)、天门冬氨酸转氨酶(AST)含量、肝脏甘油三酯(TG)、游离脂肪酸(FFA)下降,同时下调促炎介质(TNF-α、IL-6、IL-1β、MCP-1),上调抗炎因子(IL-4、IL-10)水平以及肝纤维化标志物 TGFβ 的表达, Acta2、Col1a1 和 Col1a2 被削弱。从机制上讲,CGGD 治疗改变了肠道菌群的多样性,如 、 、 、 和益生菌属的富集等所证明。结肠组织病理学结果表明 CGGD 治疗组的肠道屏障损伤得到恢复,并且与模型组相比,结肠短链脂肪酸(SCFA)特异性受体FFAR2、FFAR3和紧密连接(TJ)蛋白ZO-1、Occludin、Claudin-1的表达水平升高。进一步的代谢组学和转录组学分析表明,CGGD 通过降低 PLA2G4A、LPCAT1、COX2 和 LOX5 的水平来减轻甘油磷脂和类二十烷酸代谢紊乱引起的脂毒性。此外,CGGD还可以激活抑制性脂毒性转录因子PPARα,调节FABP1、APOC2、APOA2和LPL蛋白以促进脂肪酸分解代谢,并抑制TLR4/MyD88/NFκB通路以减轻NASH。我们的研究表明,CGGD 通过增强肠道屏障完整性和减轻 PPARα 介导的脂毒性来改善 NASH 的脂肪变性、炎症和纤维化,这使其成为 NASH 预防和治疗潜在新策略的有吸引力的候选者。

































 京公网安备 11010802027423号
京公网安备 11010802027423号