当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Depicting the Nitric Oxide (NO) Chemical Sorption in Lithium Cuprate (Li2CuO2), through Thermokinetic and Spectroscopic Analyses
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-02-01 , DOI: 10.1021/acs.jpcc.3c07018 Ramsés Martínez-Rodríguez 1 , Carlos D. Castrejón-Barrera 1 , Héctor Martínez-Hernández 1 , Fernando Plascencia-Hernández 1 , Hugo A. Lara-García 2 , Heriberto Pfeiffer 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-02-01 , DOI: 10.1021/acs.jpcc.3c07018 Ramsés Martínez-Rodríguez 1 , Carlos D. Castrejón-Barrera 1 , Héctor Martínez-Hernández 1 , Fernando Plascencia-Hernández 1 , Hugo A. Lara-García 2 , Heriberto Pfeiffer 1
Affiliation
|
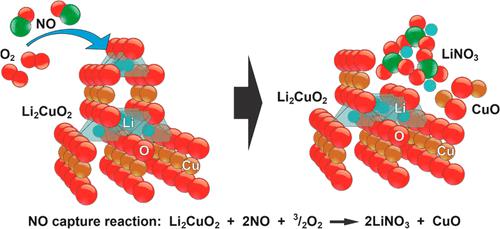
The chemisorption of NO on lithium cuprate (Li2CuO2) was studied through different techniques, including thermogravimetric and DRIFTS analyses. An initial dynamic thermogravimetric approach depicted similar efficiencies under the absence or presence oxygen (13.5 and 13.8 wt %). However, the NO capture temperature was shifted to higher values in the presence of oxygen. Moreover, the analysis of the outgoing gases showed the evolution of different nitrogen species, explained based on the Li2CuO2 reactivity, solid decompositions, and oxygen content. Then, the isothermal results showed that NO capture was improved with oxygen. Isothermal solid products were characterized, and lithium nitrate and nitrite were identified. Furthermore, a kinetic analysis was performed using two different models, showing that the presence of oxygen significantly modifies the NO sorption process. Moreover, the melting processes of lithium nitrate and nitrite enhanced the whole sorption process in the presence of oxygen; meanwhile, the oxygen absence limits the NO chemisorption.
中文翻译:

通过热动力学和光谱分析描述铜酸锂 (Li2CuO2) 中一氧化氮 (NO) 的化学吸附
通过不同的技术,包括热重分析和漂移分析,研究了NO 在铜酸锂 (Li 2 CuO 2 )上的化学吸附。初始动态热重分析方法在缺氧或存在氧气的情况下显示出相似的效率(13.5 和 13.8 wt%)。然而,在氧气存在的情况下,NO 捕获温度会转变为更高的值。此外,对排出气体的分析显示了不同氮物质的演变,这根据Li 2 CuO 2反应性、固体分解和氧含量进行了解释。然后,等温结果表明,氧气可以改善 NO 捕获。对等温固体产物进行了表征,并鉴定了硝酸锂和亚硝酸锂。此外,使用两种不同的模型进行动力学分析,表明氧气的存在显着改变了 NO 吸附过程。此外,硝酸锂和亚硝酸锂的熔化过程增强了氧气存在下的整个吸附过程;同时,缺氧限制了NO的化学吸附。
更新日期:2024-02-01
中文翻译:

通过热动力学和光谱分析描述铜酸锂 (Li2CuO2) 中一氧化氮 (NO) 的化学吸附
通过不同的技术,包括热重分析和漂移分析,研究了NO 在铜酸锂 (Li 2 CuO 2 )上的化学吸附。初始动态热重分析方法在缺氧或存在氧气的情况下显示出相似的效率(13.5 和 13.8 wt%)。然而,在氧气存在的情况下,NO 捕获温度会转变为更高的值。此外,对排出气体的分析显示了不同氮物质的演变,这根据Li 2 CuO 2反应性、固体分解和氧含量进行了解释。然后,等温结果表明,氧气可以改善 NO 捕获。对等温固体产物进行了表征,并鉴定了硝酸锂和亚硝酸锂。此外,使用两种不同的模型进行动力学分析,表明氧气的存在显着改变了 NO 吸附过程。此外,硝酸锂和亚硝酸锂的熔化过程增强了氧气存在下的整个吸附过程;同时,缺氧限制了NO的化学吸附。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号