当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solutions of Urea in tert-Butylamine: The Phenomenon of “Partial Molar Isobaric Compaction of the Solute” According to Density Measurements between 278.15 and 303.15 K at Ambient Pressure
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2024-02-01 , DOI: 10.1021/acs.jced.3c00672 Evgeniy V. Ivanov 1 , Elena Yu. Lebedeva 1 , Arina A. Pakina 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2024-02-01 , DOI: 10.1021/acs.jced.3c00672 Evgeniy V. Ivanov 1 , Elena Yu. Lebedeva 1 , Arina A. Pakina 2
Affiliation
|
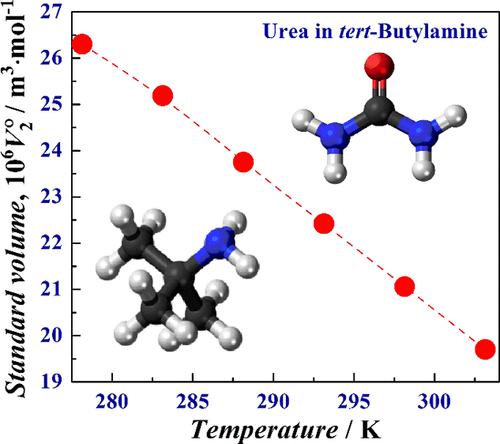
Densities of dilute solutions of urea (U) in tert-butylamine (t-BuNH2) with molalities being up to ∼0.023 mol·kg–1 were measured at T = 278.15, 283.15, 288.15, 293.15, 298.15, and 303.15 K and p = 99.6 kPa. All experiments were carried out using a sealed U-tube vibrating densimeter. The total uncertainty in the density measurements did not exceed 0.03 kg·m–3. The standard (apparent at infinite dilution) molar volumes and expansibilities of U as a solute in t-BuNH2 were computed. Similar to infinitely dilute solutions of U in methanol (MeOH) and tert-butanol (t-BuOH), the phenomenon of the so-called “negative partial molar expansibility” or “partial molar isobaric compaction of a solute” was found in the studied binary system. The mixed structural aggregate or “solvation complex” formed by a U molecule in the t-BuNH2 medium becomes increasingly compact as the temperature rises because the solute-to-solvent affinity more and more noticeably prevails over the affinity of the solvent molecules for each other. In doing so, the stronger heterocomponent H-bonding leads to the marked increase in the compactness of solvation complexes formed in U solutions going from t-BuOH to t-BuNH2.
中文翻译:

尿素在叔丁胺中的溶液:根据常压下 278.15 至 303.15 K 之间的密度测量,“溶质的部分摩尔等压压缩”现象
在T = 278.15、283.15、288.15、293.15、298.15 和 303.15 K 下测量摩尔浓度高达 ∼0.023 mol·kg –1的尿素 (U) 的叔丁胺 ( t -BuNH 2 ) 稀溶液的密度, p = 99.6 kPa。所有实验均使用密封 U 形管振动密度计进行。密度测量的总不确定度不超过0.03 kg·m –3。计算了U作为t -BuNH 2中溶质的标准摩尔体积(无限稀释时的表观摩尔体积和膨胀率) 。与U在甲醇(MeOH)和叔丁醇(t -BuOH)中的无限稀溶液类似,研究中发现了所谓的“负偏摩尔膨胀性”或“溶质的偏摩尔等压压缩”现象二进制系统。随着温度升高,U 分子在t- BuNH 2介质中形成的混合结构聚集体或“溶剂化复合物”变得越来越致密,因为溶质与溶剂的亲和力越来越明显地压倒溶剂分子对每种物质的亲和力。其他。在此过程中,更强的异组分氢键导致U溶液中从t -BuOH到t -BuNH 2形成的溶剂化络合物的致密性显着增加。
更新日期:2024-02-01
中文翻译:

尿素在叔丁胺中的溶液:根据常压下 278.15 至 303.15 K 之间的密度测量,“溶质的部分摩尔等压压缩”现象
在T = 278.15、283.15、288.15、293.15、298.15 和 303.15 K 下测量摩尔浓度高达 ∼0.023 mol·kg –1的尿素 (U) 的叔丁胺 ( t -BuNH 2 ) 稀溶液的密度, p = 99.6 kPa。所有实验均使用密封 U 形管振动密度计进行。密度测量的总不确定度不超过0.03 kg·m –3。计算了U作为t -BuNH 2中溶质的标准摩尔体积(无限稀释时的表观摩尔体积和膨胀率) 。与U在甲醇(MeOH)和叔丁醇(t -BuOH)中的无限稀溶液类似,研究中发现了所谓的“负偏摩尔膨胀性”或“溶质的偏摩尔等压压缩”现象二进制系统。随着温度升高,U 分子在t- BuNH 2介质中形成的混合结构聚集体或“溶剂化复合物”变得越来越致密,因为溶质与溶剂的亲和力越来越明显地压倒溶剂分子对每种物质的亲和力。其他。在此过程中,更强的异组分氢键导致U溶液中从t -BuOH到t -BuNH 2形成的溶剂化络合物的致密性显着增加。






























 京公网安备 11010802027423号
京公网安备 11010802027423号