当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deciphering the crystal structure of a novel nanobody against the NEIL1 DNA glycosylase
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2024-02-02 , DOI: 10.1107/s205979832400038x
Marlo K Thompson 1 , Nidhi Sharma 1 , Andrea Thorn 2 , Aishwarya Prakash 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2024-02-02 , DOI: 10.1107/s205979832400038x
Marlo K Thompson 1 , Nidhi Sharma 1 , Andrea Thorn 2 , Aishwarya Prakash 1
Affiliation
|
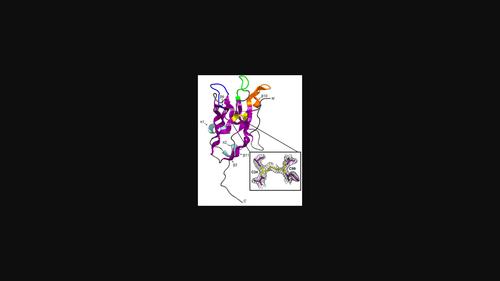
Nanobodies (VHHs) are single-domain antibodies with three antigenic CDR regions and are used in diverse scientific applications. Here, an ∼14 kDa nanobody (A5) specific for the endonuclease VIII (Nei)-like 1 or NEIL1 DNA glycosylase involved in the first step of the base-excision repair pathway was crystallized and its structure was determined to 2.1 Å resolution. The crystals posed challenges due to potential twinning and anisotropic diffraction. Despite inconclusive twinning indicators, reprocessing in an orthorhombic setting and molecular replacement in space group P21212 enabled the successful modeling of 96% of residues in the asymmetric unit, with final Rwork and Rfree values of 0.199 and 0.229, respectively.
中文翻译:

破译一种针对 NEIL1 DNA 糖基化酶的新型纳米抗体的晶体结构
纳米抗体 (VHH) 是具有三个抗原 CDR 区域的单域抗体,用于各种科学应用。在这里,结晶了对碱基切除修复途径第一步所涉及的核酸内切酶 VIII (Nei) 样 1 或 NEIL1 DNA 糖基化酶具有特异性的 ∼14 kDa 纳米抗体 (A5),并将其结构确定为 2.1 Å 分辨率。由于潜在的孪晶和各向异性衍射,晶体带来了挑战。尽管孪生指标尚无定论,但在正交环境中的再加工和空间群 P21212 中的分子替换能够成功建模不对称单元中 96% 的残基,最终 R功和 R自由值分别为 0.199 和 0.229。
更新日期:2024-02-02
中文翻译:

破译一种针对 NEIL1 DNA 糖基化酶的新型纳米抗体的晶体结构
纳米抗体 (VHH) 是具有三个抗原 CDR 区域的单域抗体,用于各种科学应用。在这里,结晶了对碱基切除修复途径第一步所涉及的核酸内切酶 VIII (Nei) 样 1 或 NEIL1 DNA 糖基化酶具有特异性的 ∼14 kDa 纳米抗体 (A5),并将其结构确定为 2.1 Å 分辨率。由于潜在的孪晶和各向异性衍射,晶体带来了挑战。尽管孪生指标尚无定论,但在正交环境中的再加工和空间群 P21212 中的分子替换能够成功建模不对称单元中 96% 的残基,最终 R功和 R自由值分别为 0.199 和 0.229。































 京公网安备 11010802027423号
京公网安备 11010802027423号