当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Kinetics of the Synthesis of Methyl Chloroacetate from Chloroacetic Acid and Methanol
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-02-02 , DOI: 10.1021/acs.iecr.3c03807 Muqian He 1 , Zhengdong Xu 1 , Xiaoping Wang 1 , Dianhua Liu 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-02-02 , DOI: 10.1021/acs.iecr.3c03807 Muqian He 1 , Zhengdong Xu 1 , Xiaoping Wang 1 , Dianhua Liu 1
Affiliation
|
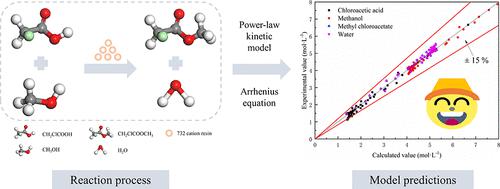
The use of sulfuric acid to catalyze the synthesis of methyl chloroacetate from chloroacetic acid and methanol leads to equipment corrosion and separation difficulties. To solve the above problems and treat chloroacetic acid efficiently, the esterification of chloroacetic acid was catalyzed with a cation exchange resin in this work. The effects of the alcohol/acid molar ratio, catalyst dosage, reaction temperature, and reaction time were investigated by single-factor and orthogonal experiments. The excellent catalytic performance was reached with 70.11% chloroacetic acid conversion under the optimal reaction conditions of 1.4:1 alcohol/acid molar ratio, 3 wt % catalyst dosage, 70 °C reaction temperature, and 2 h reaction time. In addition, a power-law kinetic model for methyl chloroacetate was developed over a cation exchange resin. The activation energy and the pre-exponential factor were calculated by the Arrhenius equation: Ea+ = 38.83 kJ/mol, Ea– = 53.64 kJ/mol, A+ = 1.71 × 105 L0.84 mol–0.84 h–1, and A– = 1.15 × 105 L0.54 mol–0.54 h–1. After verification, the kinetic model can accurately predict the esterification reaction results of chloroacetic acid and methanol under experimental conditions, which provides theoretical guidance for the simulation of the chloroacetic acid esterification process.
中文翻译:

氯乙酸与甲醇合成氯乙酸甲酯的反应动力学
采用硫酸催化氯乙酸和甲醇合成氯乙酸甲酯存在设备腐蚀和分离困难等问题。为了解决上述问题并有效处理氯乙酸,本工作采用阳离子交换树脂催化氯乙酸的酯化反应。通过单因素和正交实验考察了醇/酸摩尔比、催化剂用量、反应温度和反应时间的影响。在醇/酸摩尔比1.4:1、催化剂用量3 wt%、反应温度70 ℃、反应时间2 h的最佳反应条件下,氯乙酸转化率达到70.11%。此外,在阳离子交换树脂上开发了氯乙酸甲酯的幂律动力学模型。活化能和指前因子由阿伦尼乌斯方程计算:E a+ = 38.83 kJ/mol,E a– = 53.64 kJ/mol,A + = 1.71 × 10 5 L 0.84 mol –0.84 h –1,A – = 1.15 × 10 5 L 0.54 mol –0.54 h –1。经验证,该动力学模型能够准确预测实验条件下氯乙酸与甲醇的酯化反应结果,为氯乙酸酯化过程的模拟提供理论指导。
更新日期:2024-02-02
中文翻译:

氯乙酸与甲醇合成氯乙酸甲酯的反应动力学
采用硫酸催化氯乙酸和甲醇合成氯乙酸甲酯存在设备腐蚀和分离困难等问题。为了解决上述问题并有效处理氯乙酸,本工作采用阳离子交换树脂催化氯乙酸的酯化反应。通过单因素和正交实验考察了醇/酸摩尔比、催化剂用量、反应温度和反应时间的影响。在醇/酸摩尔比1.4:1、催化剂用量3 wt%、反应温度70 ℃、反应时间2 h的最佳反应条件下,氯乙酸转化率达到70.11%。此外,在阳离子交换树脂上开发了氯乙酸甲酯的幂律动力学模型。活化能和指前因子由阿伦尼乌斯方程计算:E a+ = 38.83 kJ/mol,E a– = 53.64 kJ/mol,A + = 1.71 × 10 5 L 0.84 mol –0.84 h –1,A – = 1.15 × 10 5 L 0.54 mol –0.54 h –1。经验证,该动力学模型能够准确预测实验条件下氯乙酸与甲醇的酯化反应结果,为氯乙酸酯化过程的模拟提供理论指导。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号