当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective synthesis of inherently chiral 9-benzylidene-9H-tribenzo[a,c,e][7]annulene and its application as a ligand platform
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-01-31 , DOI: 10.1016/j.checat.2024.100904 Xilong Wang , Chaoqin Wang , Yu Luo , Jing Li , Chunfang Gan , Shuang Luo , Qiang Zhu
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-01-31 , DOI: 10.1016/j.checat.2024.100904 Xilong Wang , Chaoqin Wang , Yu Luo , Jing Li , Chunfang Gan , Shuang Luo , Qiang Zhu
|
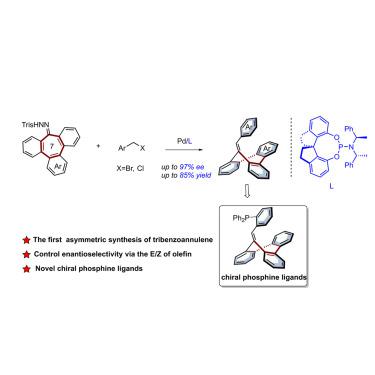
Saddle-shaped tribenzoannulene derivatives are conformationally stable and inherently chiral. The first catalytic enantioselective synthesis of this uncommon class of chiral molecules was achieved through palladium-catalyzed coupling of hydrazone derivatives of 9-tribenzo[,,][7]annulen-9-ones and benzyl bromides. The stereo-determining step in this process is the formation of the exocyclic double bond, which occurs through carbene migration insertion and β-hydride elimination. A wide range of inherently chiral 9-benzylidene-9-tribenzo[,,][7]annulenes were accessed under mild conditions in good yields with excellent enantioselectivity. A chiral phosphine ligand derived from this rigid and inherent chiral scaffold exhibited excellent asymmetric performance in Pd-catalyzed Tsuji-Trost and Rh-catalyzed 1,4-addition reactions. The reaction mechanism was confirmed by density functional theory calculations, which also shed light on the origin of the observed enantioselectivity.
中文翻译:

固有手性9-亚苄基-9H-三苯并[a,c,e][7]轮烯的对映选择性合成及其作为配体平台的应用
鞍形三苯轮烯衍生物具有构象稳定和固有的手性。这种不常见的手性分子的首次催化对映选择性合成是通过 9-三苯并[,,][7]annulen-9-ones 的腙衍生物与苄基溴的钯催化偶联实现的。该过程中的立体决定步骤是环外双键的形成,这是通过卡宾迁移插入和β-氢化物消除而发生的。在温和条件下以良好的产率和优异的对映选择性获得了多种固有手性的 9-亚苄基-9-三苯并[,,][7]轮烯。源自这种刚性且固有的手性支架的手性膦配体在 Pd 催化的 Tsuji-Trost 和 Rh 催化的 1,4-加成反应中表现出优异的不对称性能。密度泛函理论计算证实了反应机理,这也揭示了观察到的对映选择性的起源。
更新日期:2024-01-31
中文翻译:

固有手性9-亚苄基-9H-三苯并[a,c,e][7]轮烯的对映选择性合成及其作为配体平台的应用
鞍形三苯轮烯衍生物具有构象稳定和固有的手性。这种不常见的手性分子的首次催化对映选择性合成是通过 9-三苯并[,,][7]annulen-9-ones 的腙衍生物与苄基溴的钯催化偶联实现的。该过程中的立体决定步骤是环外双键的形成,这是通过卡宾迁移插入和β-氢化物消除而发生的。在温和条件下以良好的产率和优异的对映选择性获得了多种固有手性的 9-亚苄基-9-三苯并[,,][7]轮烯。源自这种刚性且固有的手性支架的手性膦配体在 Pd 催化的 Tsuji-Trost 和 Rh 催化的 1,4-加成反应中表现出优异的不对称性能。密度泛函理论计算证实了反应机理,这也揭示了观察到的对映选择性的起源。































 京公网安备 11010802027423号
京公网安备 11010802027423号