当前位置:
X-MOL 学术
›
Chem. Eng. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic nanoreactors promote GLUT1-mediated BBB permeation by generating nitric oxide for potentiating glioblastoma ferroptosis
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-30 , DOI: 10.1016/j.cej.2024.149233
Ji Liu , Mengjuan Sun , Zhen Li , Hongguang Xiang , Qiyue Wang , Xiaofei Xin , Yan Shen
Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2024-01-30 , DOI: 10.1016/j.cej.2024.149233
Ji Liu , Mengjuan Sun , Zhen Li , Hongguang Xiang , Qiyue Wang , Xiaofei Xin , Yan Shen
|
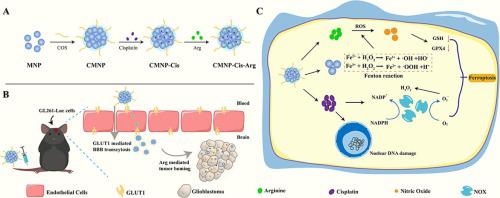
Glioblastoma, the most common malignant brain tumor with a poor prognosis, still needs to develop effective therapeutic strategies to prolong the patient’s survival. Inducing GBM tumor cell ferroptosis could be the specific assay to improve GBM therapeutic efficiency. In comparison, ferroptosis in GBM cells is limited by the low intracellular ferrous ion concentration and oxidation resistance. Herein, an MNP-based ferroptosis catalytic nanoreactor loading cisplatin (CMNP-Cis-Arg) was designed to evaluate their targeted delivery and therapeutic efficiency in GBM treatment. The ferroptosis catalytic nanoreactors achieved an effective GBM targeted transportation via chitosan oligosaccharide driven GLUT1-mediated BBB permeabilization and arginine-based tumor chemotactic. CMNP-Cis-Arg improves synergistically lipid oxidation in GBM cells by inducing ROS generation and depleting intracellular GSH. The MNPs elevate ROS levels in tumor cells by enhancing Fe2+ -based Fenton reaction and the encapsulated cisplatin as a chemotherapy agent depleting GSH via inducing the DNA strand broken. Moreover, the CMNP-Cis-Arg further induces NO biosynthesis and generates ONOO– with high oxidability to increase lipid oxidation. The anti-GBM ability of the CMNP-Cis-Arg was evaluated both in vitro and in vivo, significantly inducing the GBM tumor cells’ apoptosis and reducing the tumor burden in an orthotopic mouse model. The investigation provided a novel ferroptosis catalytic nanoreactor and confirmed their anti-GBM efficiency in boosting tumor intracellular ferroptosis, suggesting a potential therapeutic strategy worth further investigating preclinically.
中文翻译:

催化纳米反应器通过产生一氧化氮促进 GLUT1 介导的 BBB 渗透,从而增强胶质母细胞瘤铁死亡
胶质母细胞瘤是最常见的恶性脑肿瘤,预后较差,仍需要制定有效的治疗策略以延长患者的生存期。诱导GBM肿瘤细胞铁死亡可能是提高GBM治疗效率的特异性方法。相比之下,GBM细胞中的铁死亡受到细胞内亚铁离子浓度低和抗氧化性的限制。在此,设计了一种基于 MNP 的铁死亡催化纳米反应器负载顺铂 (CMNP-Cis-Arg),以评估其在 GBM 治疗中的靶向递送和治疗效率。铁死亡催化纳米反应器通过壳寡糖驱动的 GLUT1 介导的 BBB 透化和基于精氨酸的肿瘤趋化作用,实现了有效的 GBM 靶向运输。 CMNP-Cis-Arg 通过诱导 ROS 生成和消耗细胞内 GSH 来协同改善 GBM 细胞中的脂质氧化。 MNP 通过增强基于 Fe2+ 的芬顿反应来提高肿瘤细胞中的 ROS 水平,而封装的顺铂作为化疗药物通过诱导 DNA 链断裂来消耗 GSH。此外,CMNP-Cis-Arg进一步诱导NO生物合成并生成具有高氧化性的ONOO–以增加脂质氧化。在体外和体内评估了 CMNP-Cis-Arg 的抗 GBM 能力,在原位小鼠模型中显着诱导 GBM 肿瘤细胞凋亡并减轻肿瘤负荷。该研究提供了一种新型的铁死亡催化纳米反应器,并证实了它们在促进肿瘤细胞内铁死亡方面的抗 GBM 效率,提出了一种值得临床前进一步研究的潜在治疗策略。
更新日期:2024-01-30
中文翻译:

催化纳米反应器通过产生一氧化氮促进 GLUT1 介导的 BBB 渗透,从而增强胶质母细胞瘤铁死亡
胶质母细胞瘤是最常见的恶性脑肿瘤,预后较差,仍需要制定有效的治疗策略以延长患者的生存期。诱导GBM肿瘤细胞铁死亡可能是提高GBM治疗效率的特异性方法。相比之下,GBM细胞中的铁死亡受到细胞内亚铁离子浓度低和抗氧化性的限制。在此,设计了一种基于 MNP 的铁死亡催化纳米反应器负载顺铂 (CMNP-Cis-Arg),以评估其在 GBM 治疗中的靶向递送和治疗效率。铁死亡催化纳米反应器通过壳寡糖驱动的 GLUT1 介导的 BBB 透化和基于精氨酸的肿瘤趋化作用,实现了有效的 GBM 靶向运输。 CMNP-Cis-Arg 通过诱导 ROS 生成和消耗细胞内 GSH 来协同改善 GBM 细胞中的脂质氧化。 MNP 通过增强基于 Fe2+ 的芬顿反应来提高肿瘤细胞中的 ROS 水平,而封装的顺铂作为化疗药物通过诱导 DNA 链断裂来消耗 GSH。此外,CMNP-Cis-Arg进一步诱导NO生物合成并生成具有高氧化性的ONOO–以增加脂质氧化。在体外和体内评估了 CMNP-Cis-Arg 的抗 GBM 能力,在原位小鼠模型中显着诱导 GBM 肿瘤细胞凋亡并减轻肿瘤负荷。该研究提供了一种新型的铁死亡催化纳米反应器,并证实了它们在促进肿瘤细胞内铁死亡方面的抗 GBM 效率,提出了一种值得临床前进一步研究的潜在治疗策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号