当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CH3O Substitution Effect Revisited in the Vibrationally Resolved Laser-Induced Fluorescence Spectra of Methoxycyclohexoxy Radicals
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2024-02-01 , DOI: 10.1021/acs.jpca.3c07154 Yizhen Zou 1 , Fengming Yu 1 , Xiaoyu Liu 1 , Lily Zu 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2024-02-01 , DOI: 10.1021/acs.jpca.3c07154 Yizhen Zou 1 , Fengming Yu 1 , Xiaoyu Liu 1 , Lily Zu 1
Affiliation
|
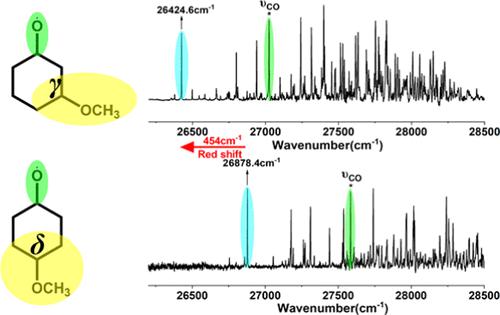
The oxy-substituted alkoxy radicals have attracted wide attention due to the increasing application of oxygenated volatile organic compounds as fuel additives and solvents. Direct detection of these intermediate radicals is desired for measuring the reaction rate and investigating the oxidation mechanism of organic compounds in the atmosphere. A charge-transfer excited state induced by CH3O substitution was identified in our previous study of 3-methoxy-1-propoxy radical [Xue, J. Phys. Chem. Chem. Phys. 2021, 23, 2586]. As the C–C bonds of chain alkoxy radicals can freely rotate, further studies are needed to understand the mechanism of this long-range charge-transfer effect. In this work, vibrational-resolved laser-induced fluorescence (LIF) spectra of 3- and 4-methoxycyclohexoxy radicals were obtained under jet-cooled conditions. A large red-shift of ∼454 cm–1 of the origin band was observed when the CH3O substituent moved from the δ site to the γ site of the cyclohexoxy radical. The LIF spectra are assigned to 3-cis (e, e) and 4-trans (e, e) conformers, respectively, with the assistance of structural optimization and electron excitation studies conducted at the CAM-B3LYP/6-311++G(d,p) level of theory. Natural transition orbital analysis reveals that the intramolecular charge transfer from the C–O–C p orbital to the radical O p orbital in 3-methoxycyclohexoxy has a strong effect on the radical CO σ → O p excitation and hence results in a spectral change. On the other hand, the spectral effect of CH3O substitution almost vanishes at δ carbon. The results propose a through-bond interaction between CH3O and radical CO groups.
中文翻译:

甲氧基环己氧基自由基振动分辨激光诱导荧光光谱中 CH3O 取代效应的再探讨
由于含氧挥发性有机化合物作为燃料添加剂和溶剂的应用不断增加,氧取代的烷氧基引起了广泛的关注。为了测量反应速率和研究大气中有机化合物的氧化机制,需要直接检测这些中间自由基。我们之前对 3-甲氧基-1-丙氧基自由基的研究中发现了由 CH 3 O 取代引起的电荷转移激发态。薛,J.物理。化学。化学。物理。 2021 , 23 , 2586]。由于链烷氧基的C-C键可以自由旋转,因此需要进一步研究来了解这种长程电荷转移效应的机制。在这项工作中,在喷射冷却条件下获得了 3- 和 4- 甲氧基环己氧基自由基的振动分辨激光诱导荧光 (LIF) 光谱。当CH 3 O取代基从环己氧基自由基的δ位点移动到γ位点时,观察到原带发生约454 cm –1的大红移。在 CAM-B3LYP/6-311++G 进行的结构优化和电子激发研究的帮助下,LIF 光谱分别分配给 3-顺式(e, e) 和 4-反式(e, e) 构象异构体(d,p) 理论水平。自然跃迁轨道分析表明,3-甲氧基环己氧基中从C–O–C p 轨道到自由基O p 轨道的分子内电荷转移对自由基CO σ → O p 激发有强烈影响,从而导致光谱变化。另一方面,CH 3 O取代的光谱效应在δ碳处几乎消失。 结果表明 CH 3 O 和自由基 CO 基团之间存在通过键相互作用。
更新日期:2024-02-01
中文翻译:

甲氧基环己氧基自由基振动分辨激光诱导荧光光谱中 CH3O 取代效应的再探讨
由于含氧挥发性有机化合物作为燃料添加剂和溶剂的应用不断增加,氧取代的烷氧基引起了广泛的关注。为了测量反应速率和研究大气中有机化合物的氧化机制,需要直接检测这些中间自由基。我们之前对 3-甲氧基-1-丙氧基自由基的研究中发现了由 CH 3 O 取代引起的电荷转移激发态。薛,J.物理。化学。化学。物理。 2021 , 23 , 2586]。由于链烷氧基的C-C键可以自由旋转,因此需要进一步研究来了解这种长程电荷转移效应的机制。在这项工作中,在喷射冷却条件下获得了 3- 和 4- 甲氧基环己氧基自由基的振动分辨激光诱导荧光 (LIF) 光谱。当CH 3 O取代基从环己氧基自由基的δ位点移动到γ位点时,观察到原带发生约454 cm –1的大红移。在 CAM-B3LYP/6-311++G 进行的结构优化和电子激发研究的帮助下,LIF 光谱分别分配给 3-顺式(e, e) 和 4-反式(e, e) 构象异构体(d,p) 理论水平。自然跃迁轨道分析表明,3-甲氧基环己氧基中从C–O–C p 轨道到自由基O p 轨道的分子内电荷转移对自由基CO σ → O p 激发有强烈影响,从而导致光谱变化。另一方面,CH 3 O取代的光谱效应在δ碳处几乎消失。 结果表明 CH 3 O 和自由基 CO 基团之间存在通过键相互作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号