当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acylation of MLKL Impacts Its Function in Necroptosis
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2024-02-01 , DOI: 10.1021/acschembio.3c00603 Apoorva J Pradhan 1 , Shweta Chitkara 1 , Ricardo X Ramirez 2 , Viviana Monje-Galvan 2 , Yasemin Sancak 3 , G Ekin Atilla-Gokcumen 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2024-02-01 , DOI: 10.1021/acschembio.3c00603 Apoorva J Pradhan 1 , Shweta Chitkara 1 , Ricardo X Ramirez 2 , Viviana Monje-Galvan 2 , Yasemin Sancak 3 , G Ekin Atilla-Gokcumen 1
Affiliation
|
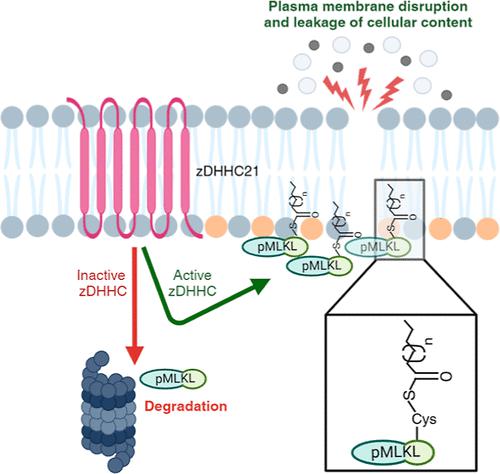
Mixed lineage kinase domain-like (MLKL) is a key signaling protein of necroptosis. Upon activation by phosphorylation, MLKL translocates to the plasma membrane and induces membrane permeabilization, which contributes to the necroptosis-associated inflammation. Membrane binding of MLKL is initially initiated by electrostatic interactions between the protein and membrane phospholipids. We previously showed that MLKL and its phosphorylated form (pMLKL) are S-acylated during necroptosis. Here, we characterize the acylation sites of MLKL and identify multiple cysteines that can undergo acylation with an interesting promiscuity at play. Our results show that MLKL and pMLKL undergo acylation at a single cysteine, with C184, C269, and C286 as possible acylation sites. Using all-atom molecular dynamic simulations, we identify differences that the acylation of MLKL causes at the protein and membrane levels. Through investigations of the S-palmitoyltransferases that might acylate pMLKL in necroptosis, we showed that zDHHC21 activity has the strongest effect on pMLKL acylation, inactivation of which profoundly reduced the pMLKL levels in cells and improved membrane integrity. These results suggest that blocking the acylation of pMLKL destabilizes the protein at the membrane interface and causes its degradation, ameliorating the necroptotic activity. At a broader level, our findings shed light on the effect of S-acylation on MLKL functioning in necroptosis and MLKL-membrane interactions mediated by its acylation.
中文翻译:

MLKL 的酰化影响其在坏死性凋亡中的功能
混合谱系激酶结构域样 (MLKL) 是坏死性凋亡的关键信号蛋白。磷酸化激活后,MLKL 易位至质膜并诱导膜透化,从而导致坏死性凋亡相关炎症。 MLKL 的膜结合最初是由蛋白质和膜磷脂之间的静电相互作用引发的。我们之前表明,MLKL 及其磷酸化形式 (pMLKL) 在坏死性凋亡过程中发生S-酰化。在这里,我们描述了 MLKL 的酰化位点,并鉴定了多个可以进行酰化的半胱氨酸,并具有有趣的混杂性。我们的结果表明,MLKL 和 pMLKL 在单个半胱氨酸上发生酰化,C184、C269 和 C286 是可能的酰化位点。通过全原子分子动力学模拟,我们确定了 MLKL 酰化在蛋白质和膜水平上引起的差异。通过对可能在坏死性凋亡中酰化 pMLKL 的S-棕榈酰转移酶的研究,我们发现 zDHHC21 活性对 pMLKL 酰化具有最强的影响,其失活显着降低了细胞中的 pMLKL 水平并改善了膜完整性。这些结果表明,阻断 pMLKL 的酰化会破坏膜界面处蛋白质的稳定性并导致其降解,从而改善坏死性凋亡活性。在更广泛的层面上,我们的研究结果揭示了S-酰化对坏死性凋亡中MLKL功能的影响以及由其酰化介导的MLKL-膜相互作用。
更新日期:2024-02-01
中文翻译:

MLKL 的酰化影响其在坏死性凋亡中的功能
混合谱系激酶结构域样 (MLKL) 是坏死性凋亡的关键信号蛋白。磷酸化激活后,MLKL 易位至质膜并诱导膜透化,从而导致坏死性凋亡相关炎症。 MLKL 的膜结合最初是由蛋白质和膜磷脂之间的静电相互作用引发的。我们之前表明,MLKL 及其磷酸化形式 (pMLKL) 在坏死性凋亡过程中发生S-酰化。在这里,我们描述了 MLKL 的酰化位点,并鉴定了多个可以进行酰化的半胱氨酸,并具有有趣的混杂性。我们的结果表明,MLKL 和 pMLKL 在单个半胱氨酸上发生酰化,C184、C269 和 C286 是可能的酰化位点。通过全原子分子动力学模拟,我们确定了 MLKL 酰化在蛋白质和膜水平上引起的差异。通过对可能在坏死性凋亡中酰化 pMLKL 的S-棕榈酰转移酶的研究,我们发现 zDHHC21 活性对 pMLKL 酰化具有最强的影响,其失活显着降低了细胞中的 pMLKL 水平并改善了膜完整性。这些结果表明,阻断 pMLKL 的酰化会破坏膜界面处蛋白质的稳定性并导致其降解,从而改善坏死性凋亡活性。在更广泛的层面上,我们的研究结果揭示了S-酰化对坏死性凋亡中MLKL功能的影响以及由其酰化介导的MLKL-膜相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号