当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Methide Affinity Scale: Key Thermodynamic Data Underpinning Catalysis, Organic Synthesis, and Organometallic and Polymer Chemistry
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2024-01-31 , DOI: 10.1021/acs.jpca.3c05974 Weam A O Altalhi 1, 2 , Bun Chan 3, 4 , Richard A J O'Hair 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2024-01-31 , DOI: 10.1021/acs.jpca.3c05974 Weam A O Altalhi 1, 2 , Bun Chan 3, 4 , Richard A J O'Hair 1
Affiliation
|
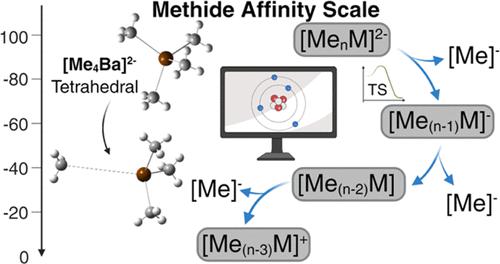
Methide transfer reactions play important roles in many areas of chemistry, including the Grignard reaction, in the transmetalation steps of metal-catalyzed cross-coupling reactions, and in the generation of cationic metal polymerization catalysts. Methide affinities (MAs) are the key thermodynamic quantity that underpin such reactions, and yet comprehensive methide affinity scales are poorly developed. Here, B3LYP-D3BJ/def2-TZVP calculations are used to calculate the energy changes (MAs) for cations (MeZ → Z+ + Me–), neutrals (MeY– → Y + Me–), and anions (MeX2– → X– + Me–) derived from permethyl species of all group s and p elements. The MAs range from 2525.8 for the singlet cation F+ to −820.4 kJ/mol for the tetramethylborate anion, Me4B–. The cations show the clearest trends: the MAs in all cases decrease going down the group, while moving across a period, the MAs increase from group 1 to group 2 and then decrease for group 3, remaining about the same or with a modest increase moving to group 4, and then continue to increase across a period to a maximum for the halogens (group 17). The anions and dianions are sensitive to hypervalency; those elements that cannot expand the octet have very unfavorable MAs (e.g., MA of Me4C requires the formation of Me5C– and of Me4B– requires the formation of Me5B2–). To address whether the anion MeY– and dianion MeZ2– are stable, the vertical detachment energies of the anions and dianions were calculated. All of the anions are thermodynamically stable with respect to electron loss, except for Me4N–, while the dianions are all thermodynamically unstable with respect to electron loss. The kinetic stability of the dianions with respect to methide and electron loss was also evaluated for the lowest MAs. The only dianions that might be kinetically stable and observable in the gas phase are Me4Ca2–, Me4Sr2–, and Me4Ba2–. The dianion CF3CaF32– is predicted to be both thermodynamically and kinetically stable in the gas phase.
中文翻译:

甲基化物亲和量表:支撑催化、有机合成、有机金属和聚合物化学的关键热力学数据
甲基化物转移反应在许多化学领域发挥着重要作用,包括格氏反应、金属催化交叉偶联反应的金属转移步骤以及阳离子金属聚合催化剂的生成。甲基化物亲和力(MA)是支撑此类反应的关键热力学量,但全面的甲基化物亲和力尺度尚不完善。此处,B3LYP-D3BJ/def2-TZVP 计算用于计算阳离子 (MeZ → Z + + Me – )、中性离子 (MeY – → Y + Me – ) 和阴离子 (MeX 2– → X – + Me – ) 衍生自所有 s 和 p 族元素的全甲基物质。 MA 范围从单线态阳离子 F +的 2525.8 到四甲基硼酸根阴离子 Me 4 B – 的-820.4 kJ/mol。阳离子显示出最明显的趋势:在所有情况下,MA 在组中向下移动,而在一段时期内移动时,MA 从组 1 增加到组 2,然后在组 3 中减少,保持大致相同或略有增加到第 4 组,然后在一段时间内继续增加到卤素的最大值(第 17 组)。阴离子和双阴离子对高价态敏感;那些不能扩展八位组的元素具有非常不利的MA(例如,Me 4 C 的MA 需要形成Me 5 C –而Me 4 B –需要形成Me 5 B 2– )。为了确定阴离子 MeY –和二价阴离子 MeZ 2 –是否稳定,计算了阴离子和二价阴离子的垂直脱离能。 除了 Me 4 N –之外,所有阴离子对于电子损失都是热力学稳定的,而二价阴离子对于电子损失都是热力学不稳定的。还针对最低 MA 评估了二价阴离子相对于甲基化物和电子损失的动力学稳定性。唯一可能在气相中动力学稳定且可观察到的二价阴离子是 Me 4 Ca 2– 、Me 4 Sr 2–和 Me 4 Ba 2– 。二价阴离子CF 3 CaF 3 2–预计在气相中具有热力学和动力学稳定性。
更新日期:2024-01-31
中文翻译:

甲基化物亲和量表:支撑催化、有机合成、有机金属和聚合物化学的关键热力学数据
甲基化物转移反应在许多化学领域发挥着重要作用,包括格氏反应、金属催化交叉偶联反应的金属转移步骤以及阳离子金属聚合催化剂的生成。甲基化物亲和力(MA)是支撑此类反应的关键热力学量,但全面的甲基化物亲和力尺度尚不完善。此处,B3LYP-D3BJ/def2-TZVP 计算用于计算阳离子 (MeZ → Z + + Me – )、中性离子 (MeY – → Y + Me – ) 和阴离子 (MeX 2– → X – + Me – ) 衍生自所有 s 和 p 族元素的全甲基物质。 MA 范围从单线态阳离子 F +的 2525.8 到四甲基硼酸根阴离子 Me 4 B – 的-820.4 kJ/mol。阳离子显示出最明显的趋势:在所有情况下,MA 在组中向下移动,而在一段时期内移动时,MA 从组 1 增加到组 2,然后在组 3 中减少,保持大致相同或略有增加到第 4 组,然后在一段时间内继续增加到卤素的最大值(第 17 组)。阴离子和双阴离子对高价态敏感;那些不能扩展八位组的元素具有非常不利的MA(例如,Me 4 C 的MA 需要形成Me 5 C –而Me 4 B –需要形成Me 5 B 2– )。为了确定阴离子 MeY –和二价阴离子 MeZ 2 –是否稳定,计算了阴离子和二价阴离子的垂直脱离能。 除了 Me 4 N –之外,所有阴离子对于电子损失都是热力学稳定的,而二价阴离子对于电子损失都是热力学不稳定的。还针对最低 MA 评估了二价阴离子相对于甲基化物和电子损失的动力学稳定性。唯一可能在气相中动力学稳定且可观察到的二价阴离子是 Me 4 Ca 2– 、Me 4 Sr 2–和 Me 4 Ba 2– 。二价阴离子CF 3 CaF 3 2–预计在气相中具有热力学和动力学稳定性。































 京公网安备 11010802027423号
京公网安备 11010802027423号