当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Early Process Development of PF-07054894, a Squaramide-Based Antagonist of C–C Chemokine Receptor Type 6 (CCR6)
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-01-30 , DOI: 10.1021/acs.oprd.3c00451 Gary M. Chinigo , Emma L. McInturff , Scott W. Bagley , Richard W. Barnhart , David C. Blakemore , Lu Han , Taegyo Lee , Javier Magano , J. Christopher McWilliams , Sebastien Monfette , James J. Mousseau , Senliang Pan , Dylan Pedro , Hahdi H. Perfect , Jeffrey W. Raggon , Peter R. Rose , John Sagal , John I. Trujillo , Jared Van Haitsma , Michael G. Vetelino , Xiaojing Helen Yang
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-01-30 , DOI: 10.1021/acs.oprd.3c00451 Gary M. Chinigo , Emma L. McInturff , Scott W. Bagley , Richard W. Barnhart , David C. Blakemore , Lu Han , Taegyo Lee , Javier Magano , J. Christopher McWilliams , Sebastien Monfette , James J. Mousseau , Senliang Pan , Dylan Pedro , Hahdi H. Perfect , Jeffrey W. Raggon , Peter R. Rose , John Sagal , John I. Trujillo , Jared Van Haitsma , Michael G. Vetelino , Xiaojing Helen Yang
|
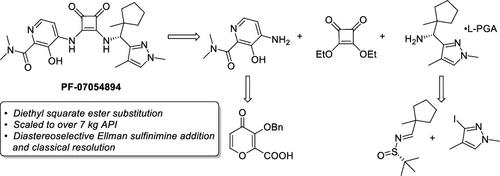
The original synthesis of a CCR6 antagonist and subsequent enablement for kilogram manufacture is presented. Highlighted improvements made in the preparation for the scale-up campaign include (1) a refined route to access a key iodopyrazole which previously suffered from low yield due to poor regioselectivity, (2) optimization of a sulfinimine addition reaction to form a sterically congested amine with high stereocontrol, (3) implementation of a route to an aminopyridine fragment, and (4) efficient construction of the target molecule by sequential substitution of diethyl squarate with two elaborated amines. These enablement efforts have resulted in the successful preparation of >7 kg of crystalline API to perform early toxicological studies and initiate Phase 1 clinical trials.
中文翻译:

PF-07054894 的早期工艺开发,一种基于方酰胺的 C-C 趋化因子受体 6 型 (CCR6) 拮抗剂
介绍了 CCR6 拮抗剂的原始合成以及随后的公斤级生产。在放大生产准备工作中取得的突出改进包括(1)获得关键碘吡唑的精细路线,该路线之前由于区域选择性差而收率低,(2)优化亚磺亚胺加成反应以形成空间拥挤的胺具有高度立体控制,(3)实现氨基吡啶片段的路线,以及(4)通过用两种精心设计的胺顺序取代方酸二乙酯来有效构建目标分子。这些支持工作已成功制备 >7 kg 的结晶 API,用于进行早期毒理学研究并启动 1 期临床试验。
更新日期:2024-01-30
中文翻译:

PF-07054894 的早期工艺开发,一种基于方酰胺的 C-C 趋化因子受体 6 型 (CCR6) 拮抗剂
介绍了 CCR6 拮抗剂的原始合成以及随后的公斤级生产。在放大生产准备工作中取得的突出改进包括(1)获得关键碘吡唑的精细路线,该路线之前由于区域选择性差而收率低,(2)优化亚磺亚胺加成反应以形成空间拥挤的胺具有高度立体控制,(3)实现氨基吡啶片段的路线,以及(4)通过用两种精心设计的胺顺序取代方酸二乙酯来有效构建目标分子。这些支持工作已成功制备 >7 kg 的结晶 API,用于进行早期毒理学研究并启动 1 期临床试验。















































 京公网安备 11010802027423号
京公网安备 11010802027423号