当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Dichloroisoeverninic Acid Biosynthesis and Chemoenzymatic Synthesis of New Orthosomycins
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2024-01-30 , DOI: 10.1021/acschembio.3c00693 Audrey E Yñigez-Gutierrez 1 , Jennifer E Wurm 1, 2 , Jordan T Froese 1 , Nicholas E Rosenthal 1 , Brian O Bachmann 1
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2024-01-30 , DOI: 10.1021/acschembio.3c00693 Audrey E Yñigez-Gutierrez 1 , Jennifer E Wurm 1, 2 , Jordan T Froese 1 , Nicholas E Rosenthal 1 , Brian O Bachmann 1
Affiliation
|
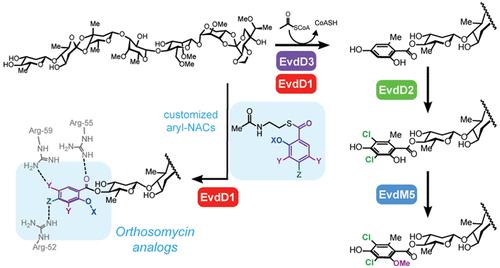
The orthosomycins are highly modified oligosaccharide natural products with a broad spectrum and potent antimicrobial activities. These include everninomicins and avilamycins, which inhibit protein translation by binding a unique site on the bacterial ribosome. Notably, ribosomal bound structures reveal a network of interactions between the 50S subunit and dichloroisoeverninic acid (DCIE), the aromatic A1-ring conserved across orthosomycins, but the relationship of these interactions to their antimicrobial activity remains undetermined. Genetic functional analysis of three genes putatively associated with DCIE biosynthesis in the everninomicin producer Micromonospora carbonacea delineates the native biosynthetic pathway and provides previously unreported advanced biosynthetic intermediates. Subsequent in vitro biochemical analyses demonstrate the complete DCIE biosynthetic pathway and provide access to novel everninomicin analogs. In addition to the orsellinate synthase EvdD3 and a flavin-dependent halogenase EvdD2, our results identified a key acyltransferase, EvdD1, responsible for transferring orsellinate from the acyl carrier protein domain of EvdD3 to a heptasaccharide orthosomycin biosynthetic intermediate. We have also shown that EvdD1 is able to transfer unnatural aryl groups via their N-acyl cysteamine thioesters to the everninomicin scaffold and used this as a biocatalyst to generate a panel of unnatural aryl analogs. The impact of diverse aryl functional group substitution on both ribosome inhibition and antibacterial activities demonstrates the importance of the DCIE moiety in the pharmacology of orthosomycins, notably revealing an uncoupling between ribosomal engagement and antibiotic activity. Control of A1-ring functionality in this class of molecules provides a potential handle to explore and address pharmacological roles of the DCIE ring in this potent and unique class of antibiotics.
中文翻译:

新型正霉素的二氯异维宁酸生物合成和化学酶法合成的表征
正霉素是高度修饰的低聚糖天然产物,具有广谱和有效的抗菌活性。其中包括everninomicins和avilamycin,它们通过结合细菌核糖体上的独特位点来抑制蛋白质翻译。值得注意的是,核糖体结合结构揭示了 50S 亚基和二氯异戊酸 (DCIE)(在正霉素中保守的芳香 A 1环)之间的相互作用网络,但这些相互作用与其抗菌活性的关系仍未确定。对万尼霉素生产者碳小单孢菌中推测与 DCIE 生物合成相关的三个基因的遗传功能分析描绘了天然生物合成途径,并提供了先前未报道的高级生物合成中间体。随后的体外生化分析证明了完整的 DCIE 生物合成途径,并提供了新型 Everninomicin 类似物的途径。除了奥赛林酸合酶 EvdD3 和黄素依赖性卤化酶 EvdD2 之外,我们的结果还鉴定了一种关键的酰基转移酶 EvdD1,它负责将奥赛林酸从 EvdD3 的酰基载体蛋白结构域转移到七糖正霉素生物合成中间体。我们还表明,EvdD1 能够通过其N-酰基半胱胺硫酯将非天然芳基转移到 Everninomicin 支架上,并将其用作生物催化剂来生成一组非天然芳基类似物。不同芳基官能团取代对核糖体抑制和抗菌活性的影响证明了 DCIE 部分在正霉素药理学中的重要性,特别揭示了核糖体结合和抗生素活性之间的解偶联。 此类分子中A 1环功能性的控制为探索和解决DCIE环在此类有效且独特的抗生素中的药理学作用提供了潜在的途径。
更新日期:2024-01-30
中文翻译:

新型正霉素的二氯异维宁酸生物合成和化学酶法合成的表征
正霉素是高度修饰的低聚糖天然产物,具有广谱和有效的抗菌活性。其中包括everninomicins和avilamycin,它们通过结合细菌核糖体上的独特位点来抑制蛋白质翻译。值得注意的是,核糖体结合结构揭示了 50S 亚基和二氯异戊酸 (DCIE)(在正霉素中保守的芳香 A 1环)之间的相互作用网络,但这些相互作用与其抗菌活性的关系仍未确定。对万尼霉素生产者碳小单孢菌中推测与 DCIE 生物合成相关的三个基因的遗传功能分析描绘了天然生物合成途径,并提供了先前未报道的高级生物合成中间体。随后的体外生化分析证明了完整的 DCIE 生物合成途径,并提供了新型 Everninomicin 类似物的途径。除了奥赛林酸合酶 EvdD3 和黄素依赖性卤化酶 EvdD2 之外,我们的结果还鉴定了一种关键的酰基转移酶 EvdD1,它负责将奥赛林酸从 EvdD3 的酰基载体蛋白结构域转移到七糖正霉素生物合成中间体。我们还表明,EvdD1 能够通过其N-酰基半胱胺硫酯将非天然芳基转移到 Everninomicin 支架上,并将其用作生物催化剂来生成一组非天然芳基类似物。不同芳基官能团取代对核糖体抑制和抗菌活性的影响证明了 DCIE 部分在正霉素药理学中的重要性,特别揭示了核糖体结合和抗生素活性之间的解偶联。 此类分子中A 1环功能性的控制为探索和解决DCIE环在此类有效且独特的抗生素中的药理学作用提供了潜在的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号