Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2024-01-29 , DOI: 10.1016/j.xcrp.2024.101786 Woo Hee Kim , Seon Bin Song , Da Eun Lee , Prithwish Goswami , You Kyoung Chung , Sohyeong Choi , Won Hee Jung , Sang Un Choi , Shinwon Ham , Yujin Oh , Ki Hyun Kim , Joonsuk Huh , Han Yong Bae
|
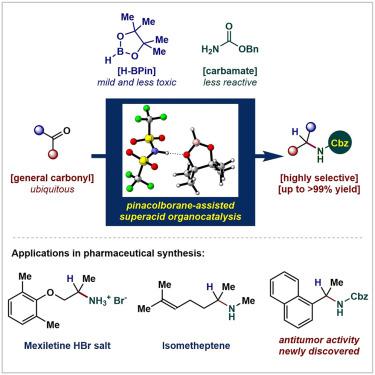
α-Secondary alkyl amines are structural motifs frequently encountered in a wide variety of natural products and pharmaceuticals. The N-benzyloxycarbonyl (Cbz) compound is a widely used precursor, acknowledged for its efficacy in implementing a masked amine strategy to access a privileged moiety. Although reductive amination is conducted as a crucial portion of the pharmaceutical industry, direct catalytic access to alkyl Cbz-amine is still rare due to the low reactivity of carbamate. Here, we show a superacid organocatalyst enabled direct access to bioactive Cbz-protected α-secondary alkyl amines using general ketones as the starting material. Through the highly selective and robust catalytic process, a wide substrate scope including drug precursor scaffolds in preparative scalability (up to >99% yield) with practical pharmaceutical syntheses is achieved. The obtained N-Cbz products are found to possess strong cytotoxicities in in vitro bioactivity evaluations, indicating their potential as promising candidates for new anticancer drug discovery.
中文翻译:

频哪醇硼烷辅助的超酸有机催化能够直接获得细胞毒性烷基 N-Cbz 胺
α-仲烷基胺是各种天然产物和药物中经常遇到的结构基序。N-苄氧基羰基 (Cbz) 化合物是一种广泛使用的前体,因其在实施掩蔽胺策略以获得特权部分方面的功效而受到认可。尽管还原胺化是制药工业的重要组成部分,但由于氨基甲酸酯的反应活性较低,直接催化获得烷基Cbz-胺仍然很少。在这里,我们展示了一种超强酸有机催化剂,能够使用一般酮作为起始材料直接获得生物活性 Cbz 保护的 α-仲烷基胺。通过高度选择性和稳健的催化过程,实现了广泛的底物范围,包括具有制备可扩展性(高达> 99%产率)的药物前体支架和实用的药物合成。在体外生物活性评价中发现所获得的N -Cbz 产品具有很强的细胞毒性,表明它们有可能成为新抗癌药物发现的有希望的候选者。






























 京公网安备 11010802027423号
京公网安备 11010802027423号