当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanodrugs mediate TAMs-related arginine metabolism interference to boost photodynamic immunotherapy
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-01-29 , DOI: 10.1016/j.jconrel.2024.01.045 Yi Chen 1 , Xian Shu 1 , Jia-Yi Guo 1 , Yun Xiang 1 , Shi-Yu Liang 2 , Jin-Mei Lai 2 , Jia-Yi Zhou 1 , Li-Han Liu 2 , Ping Wang 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2024-01-29 , DOI: 10.1016/j.jconrel.2024.01.045 Yi Chen 1 , Xian Shu 1 , Jia-Yi Guo 1 , Yun Xiang 1 , Shi-Yu Liang 2 , Jin-Mei Lai 2 , Jia-Yi Zhou 1 , Li-Han Liu 2 , Ping Wang 1
Affiliation
|
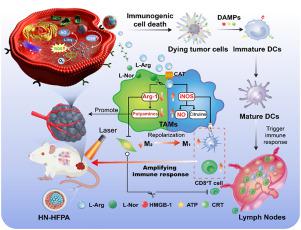
As a potential treatment strategy for low immunogenic triple negative breast cancer (TNBC), photodynamic therapy (PDT) induced antitumor immunotherapy is greatly limited by the immunosuppressive tumor microenvironment (ITM), especially the M2 phenotype tumor-associated macrophages (TAMs). The balance of arginine metabolism plays an important role in TAMs polarization. Herein, a multifunctional nanoplatform (defined as HN-HFPA) was employed to burst the anti-tumor immunity of TNBC post PDT by reeducating TAMs through interfering the TAMs-associated arginine metabolism. The -arginine (-Arg) was loaded in the hollow cavity of HN-HFPA, which could not only generate nitric oxide (NO) for tumor therapy, but also serve as a substrate of arginine metabolism pathway. As an inhibitor of arginases-1 (Arg-1) of M2 TAMs, -norvaline (-Nor) was modified to the hyaluronic acid (HA), and coated in the surface of HFPA. After degradation of HA by hyaluronidase in tumor tissue and GSH-mediated disintegration, HN-HFPA depleted intracellular GSH, produced remarkable reactive oxygen species (ROS) under light irradiation and released -Arg to generate NO, which induced tumor immunogenic cell death (ICD). Real-time ultrasound imaging of tumor was realized taking advantage of the gas feature of NO. The -Nor suppressed the Arg-1 overexpressed in M2, which skewed the balance of arginine metabolism and reversed the ITM with increased ratios of M1 and CD8 T cells, finally resulted in amplified antitumor immune response and apparent tumor metastasis inhibition. This study remodeled ITM to strengthen immune response post PDT, which provided a promising treatment strategy for TNBC.
中文翻译:

纳米药物介导 TAM 相关精氨酸代谢干扰以促进光动力免疫治疗
作为低免疫原性三阴性乳腺癌(TNBC)的潜在治疗策略,光动力疗法(PDT)诱导的抗肿瘤免疫治疗受到免疫抑制肿瘤微环境(ITM),特别是M2表型肿瘤相关巨噬细胞(TAM)的极大限制。精氨酸代谢的平衡在TAMs极化中起着重要作用。在此,采用多功能纳米平台(定义为 HN-HFPA)通过干扰 TAM 相关的精氨酸代谢来重新训练 TAM,从而在 PDT 后爆发 TNBC 的抗肿瘤免疫力。将精氨酸(-Arg)负载在HN-HFPA的空腔中,不仅可以产生用于肿瘤治疗的一氧化氮(NO),而且可以作为精氨酸代谢途径的底物。作为M2 TAMs的精氨酸酶-1(Arg-1)抑制剂,将-正缬氨酸(-Nor)修饰为透明质酸(HA),并涂覆在HFPA表面。 HN-HFPA 经肿瘤组织中的透明质酸酶降解和 GSH 介导的崩解后,耗尽细胞内的 GSH,在光照射下产生显着的活性氧 (ROS) 并释放 -Arg 生成 NO,从而诱导肿瘤免疫原性细胞死亡 (ICD) 。利用NO的气体特性实现了肿瘤的实时超声成像。 -Nor抑制了M2中过度表达的Arg-1,从而扭曲了精氨酸代谢的平衡,并随着M1和CD8 T细胞比例的增加而逆转了ITM,最终导致抗肿瘤免疫反应增强和明显的肿瘤转移抑制。这项研究重塑了 ITM 以增强 PDT 后的免疫反应,这为 TNBC 提供了一种有前途的治疗策略。
更新日期:2024-01-29
中文翻译:

纳米药物介导 TAM 相关精氨酸代谢干扰以促进光动力免疫治疗
作为低免疫原性三阴性乳腺癌(TNBC)的潜在治疗策略,光动力疗法(PDT)诱导的抗肿瘤免疫治疗受到免疫抑制肿瘤微环境(ITM),特别是M2表型肿瘤相关巨噬细胞(TAM)的极大限制。精氨酸代谢的平衡在TAMs极化中起着重要作用。在此,采用多功能纳米平台(定义为 HN-HFPA)通过干扰 TAM 相关的精氨酸代谢来重新训练 TAM,从而在 PDT 后爆发 TNBC 的抗肿瘤免疫力。将精氨酸(-Arg)负载在HN-HFPA的空腔中,不仅可以产生用于肿瘤治疗的一氧化氮(NO),而且可以作为精氨酸代谢途径的底物。作为M2 TAMs的精氨酸酶-1(Arg-1)抑制剂,将-正缬氨酸(-Nor)修饰为透明质酸(HA),并涂覆在HFPA表面。 HN-HFPA 经肿瘤组织中的透明质酸酶降解和 GSH 介导的崩解后,耗尽细胞内的 GSH,在光照射下产生显着的活性氧 (ROS) 并释放 -Arg 生成 NO,从而诱导肿瘤免疫原性细胞死亡 (ICD) 。利用NO的气体特性实现了肿瘤的实时超声成像。 -Nor抑制了M2中过度表达的Arg-1,从而扭曲了精氨酸代谢的平衡,并随着M1和CD8 T细胞比例的增加而逆转了ITM,最终导致抗肿瘤免疫反应增强和明显的肿瘤转移抑制。这项研究重塑了 ITM 以增强 PDT 后的免疫反应,这为 TNBC 提供了一种有前途的治疗策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号