Toxicology and Applied Pharmacology ( IF 3.3 ) Pub Date : 2024-01-23 , DOI: 10.1016/j.taap.2024.116835 Ai-Cheng Wang 1 , Xiao-Ming Qi 1 , Qing-Fang Li 1 , Yi-Jia Feng 1 , Yuan-Lin Zhang 1 , Hui-Zhi Wei 2 , Jin-Shan Li 1 , Yuan-Biao Qiao 1 , Qing-Shan Li 2
|
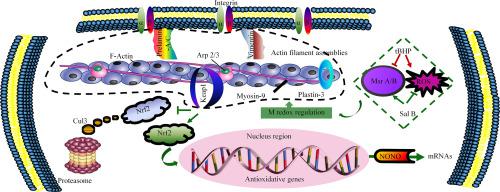
Actin-interacting proteins are important molecules for filament assembly and cytoskeletal signaling within vascular endothelium. Disruption in their interactions causes endothelial pathogenesis through redox imbalance. Actin filament redox regulation remains largely unexplored, in the context of pharmacological treatment. This work focused on the peptidyl methionine (M) redox regulation of actin-interacting proteins, aiming at elucidating its role on governing antioxidative signaling and response. Endothelial EA.hy926 cells were subjected to treatment with salvianolic acid B (Sal B) and tert-butyl-hydroperoxide (tBHP) stimulation. Mass spectrometry was employed to characterize redox status of proteins, including actin, myosin-9, kelch-like erythroid-derived cap-n-collar homology-associated protein 1 (Keap1), plastin-3, prelamin-A/C and vimentin. The protein redox landscape revealed distinct stoichiometric ratios or reaction site transitions mediated by M sulfoxide reductase and reactive oxygen species. In comparison with effects of tBHP stimulation, Sal B treatment prevented oxidation at actin M325, myosin-9 M1489/1565, Keap1 M120, plastin-3 M592, prelamin-A/C M187/371/540 and vimentin M344. For Keap1, reaction site was transitioned within its scaffolding region to the actin ring. These protein M oxidation regulations contributed to the Sal B cytoprotective effects on actin filament. Additionally, regarding the Keap1 homo-dimerization region, Sal B preventive roles against M120 oxidation acted as a primary signal driver to activate nuclear factor erythroid 2-related factor 2 (Nrf2). Transcriptional splicing of non-POU domain-containing octamer-binding protein was validated during the Sal B-mediated overexpression of NAD(P)H dehydrogenase [quinone] 1. This molecular redox regulation of actin-interacting proteins provided valuable insights into the phenolic structures of Sal B analogs, showing potential antioxidative effects on vascular endothelium.
中文翻译:

肌动蛋白相互作用蛋白的蛋氨酸氧化还原调节主要控制 EA.hy926 细胞中的抗氧化信号传导和对丹酚酸 B 处理的反应
肌动蛋白相互作用蛋白是血管内皮内丝组装和细胞骨架信号传导的重要分子。它们相互作用的破坏通过氧化还原失衡导致内皮发病机制。在药物治疗的背景下,肌动蛋白丝氧化还原调节在很大程度上仍未被探索。这项工作重点关注肌动蛋白相互作用蛋白的肽基甲硫氨酸(M)氧化还原调节,旨在阐明其在控制抗氧化信号和反应中的作用。内皮 EA.hy926 细胞接受丹酚酸 B (Sal B) 和叔丁基过氧化氢 (tBHP) 刺激处理。采用质谱法表征蛋白质的氧化还原状态,包括肌动蛋白、肌球蛋白-9、kelch 样红系衍生的 cap-n-collar 同源相关蛋白 1 (Keap1)、plastin-3、prelamin-A/C 和波形蛋白。蛋白质氧化还原景观揭示了由 M 亚砜还原酶和活性氧介导的不同化学计量比或反应位点转变。与 tBHP 刺激的效果相比,Sal B 处理可防止肌动蛋白 M325、肌球蛋白-9 M1489/1565、Keap1 M120、plastin-3 M592、prelamin-A/C M187/371/540 和波形蛋白 M344 的氧化。对于 Keap1,反应位点在其支架区域内转移至肌动蛋白环。这些蛋白质 M 氧化调节有助于 Sal B 对肌动蛋白丝的细胞保护作用。此外,对于 Keap1 同源二聚化区域,Sal B 对 M120 氧化的预防作用是激活核因子红细胞 2 相关因子 2 (Nrf2) 的主要信号驱动因素。 在 Sal B 介导的 NAD( P )H 脱氢酶 [醌] 1 过表达过程中,验证了包含非 POU 结构域的八聚体结合蛋白的转录剪接。肌动蛋白相互作用蛋白的这种分子氧化还原调节为酚类结构提供了有价值的见解Sal B 类似物,显示出对血管内皮的潜在抗氧化作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号