当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Methods Enable the Prediction of Improved Catalysts for Nickel-Catalyzed Cross-Electrophile Coupling
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-26 , DOI: 10.1021/jacs.3c09554
Michelle E Akana 1 , Sergei Tcyrulnikov 2 , Brett D Akana-Schneider 1 , Giselle P Reyes 2 , Sebastien Monfette 2 , Matthew S Sigman 3 , Eric C Hansen 2 , Daniel J Weix 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-26 , DOI: 10.1021/jacs.3c09554
Michelle E Akana 1 , Sergei Tcyrulnikov 2 , Brett D Akana-Schneider 1 , Giselle P Reyes 2 , Sebastien Monfette 2 , Matthew S Sigman 3 , Eric C Hansen 2 , Daniel J Weix 1
Affiliation
|
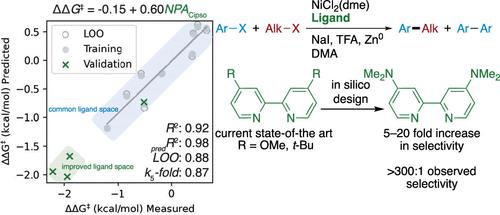
Cross-electrophile coupling has emerged as an attractive and efficient method for the synthesis of C(sp2)–C(sp3) bonds. These reactions are most often catalyzed by nickel complexes of nitrogenous ligands, especially 2,2′-bipyridines. Precise prediction, selection, and design of optimal ligands remains challenging, despite significant increases in reaction scope and mechanistic understanding. Molecular parameterization and statistical modeling provide a path to the development of improved bipyridine ligands that will enhance the selectivity of existing reactions and broaden the scope of electrophiles that can be coupled. Herein, we describe the generation of a computational ligand library, correlation of observed reaction outcomes with features of the ligands, and the in silico design of improved bipyridine ligands for Ni-catalyzed cross-electrophile coupling. The new nitrogen-substituted ligands display a 5-fold increase in selectivity for product formation versus homodimerization when compared to the current state of the art. This increase in selectivity and yield was general for several cross-electrophile couplings, including the challenging coupling of an aryl chloride with an N-alkylpyridinium salt.
中文翻译:

计算方法可以预测镍催化交叉电偶联催化剂的改进
交叉亲电子偶联已成为合成 C(sp 2 )–C(sp 3 ) 键的一种有吸引力且有效的方法。这些反应最常由含氮配体的镍络合物催化,尤其是 2,2'-联吡啶。尽管反应范围和机理理解显着增加,但最佳配体的精确预测、选择和设计仍然具有挑战性。分子参数化和统计模型为开发改进的联吡啶配体提供了一条途径,这将增强现有反应的选择性并扩大可偶联的亲电子试剂的范围。在此,我们描述了计算配体库的生成、观察到的反应结果与配体特征的相关性,以及用于镍催化交叉亲电子偶联的改进联吡啶配体的计算机设计。与现有技术相比,新的氮取代配体显示出与同二聚化相比,产物形成的选择性提高了 5 倍。这种选择性和产率的增加对于几种交叉亲电试剂偶联来说是普遍存在的,包括芳基氯与N-烷基吡啶鎓盐的具有挑战性的偶联。
更新日期:2024-01-26
中文翻译:

计算方法可以预测镍催化交叉电偶联催化剂的改进
交叉亲电子偶联已成为合成 C(sp 2 )–C(sp 3 ) 键的一种有吸引力且有效的方法。这些反应最常由含氮配体的镍络合物催化,尤其是 2,2'-联吡啶。尽管反应范围和机理理解显着增加,但最佳配体的精确预测、选择和设计仍然具有挑战性。分子参数化和统计模型为开发改进的联吡啶配体提供了一条途径,这将增强现有反应的选择性并扩大可偶联的亲电子试剂的范围。在此,我们描述了计算配体库的生成、观察到的反应结果与配体特征的相关性,以及用于镍催化交叉亲电子偶联的改进联吡啶配体的计算机设计。与现有技术相比,新的氮取代配体显示出与同二聚化相比,产物形成的选择性提高了 5 倍。这种选择性和产率的增加对于几种交叉亲电试剂偶联来说是普遍存在的,包括芳基氯与N-烷基吡啶鎓盐的具有挑战性的偶联。

































 京公网安备 11010802027423号
京公网安备 11010802027423号