当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron Heme Enzyme-Catalyzed Cyclopropanations with Diazirines as Carbene Precursors: Computational Explorations of Diazirine Activation and Cyclopropanation Mechanism
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-25 , DOI: 10.1021/jacs.3c06030 Torben Rogge 1 , Qingyang Zhou 1 , Nicholas J Porter 2 , Frances H Arnold 2 , K N Houk 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2024-01-25 , DOI: 10.1021/jacs.3c06030 Torben Rogge 1 , Qingyang Zhou 1 , Nicholas J Porter 2 , Frances H Arnold 2 , K N Houk 1
Affiliation
|
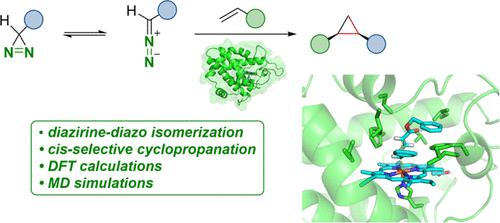
The mechanism of cyclopropanations with diazirines as air-stable and user-friendly alternatives to commonly employed diazo compounds within iron heme enzyme-catalyzed carbene transfer reactions has been studied by means of density functional theory (DFT) calculations of model systems, quantum mechanics/molecular mechanics (QM/MM) calculations, and molecular dynamics (MD) simulations of the iron carbene and the cyclopropanation transition state in the enzyme active site. The reaction is initiated by a direct diazirine-diazo isomerization occurring in the active site of the enzyme. In contrast, an isomerization mechanism proceeding via the formation of a free carbene intermediate in lieu of a direct, one-step isomerization process was observed for model systems. Subsequent reaction with benzyl acrylate takes place through stepwise C–C bond formation via a diradical intermediate, delivering the cyclopropane product. The origin of the observed diastereo- and enantioselectivity in the enzyme was investigated through MD simulations, which indicate a preferred formation of the cis-cyclopropane by steric control.
中文翻译:

铁血红素酶催化二氮丙烷作为卡宾前体的环丙烷化:二氮丙烷活化和环丙烷化机制的计算探索
通过模型系统的密度泛函理论 (DFT) 计算、量子力学/分子研究了铁血红素酶催化卡宾转移反应中常用重氮化合物的空气稳定且用户友好的替代物二氮杂环丙烷化的机理。铁卡宾和酶活性位点的环丙烷化过渡态的力学(QM/MM)计算和分子动力学(MD)模拟。该反应由酶活性位点发生的直接二氮丙啶-重氮异构化引发。相比之下,在模型系统中观察到异构化机制是通过形成游离卡宾中间体来代替直接的一步异构化过程。随后通过双自由基中间体逐步形成 C-C 键与丙烯酸苄酯发生反应,生成环丙烷产物。通过MD模拟研究了酶中观察到的非对映选择性和对映选择性的起源,这表明通过空间控制优选形成顺式环丙烷。
更新日期:2024-01-25
中文翻译:

铁血红素酶催化二氮丙烷作为卡宾前体的环丙烷化:二氮丙烷活化和环丙烷化机制的计算探索
通过模型系统的密度泛函理论 (DFT) 计算、量子力学/分子研究了铁血红素酶催化卡宾转移反应中常用重氮化合物的空气稳定且用户友好的替代物二氮杂环丙烷化的机理。铁卡宾和酶活性位点的环丙烷化过渡态的力学(QM/MM)计算和分子动力学(MD)模拟。该反应由酶活性位点发生的直接二氮丙啶-重氮异构化引发。相比之下,在模型系统中观察到异构化机制是通过形成游离卡宾中间体来代替直接的一步异构化过程。随后通过双自由基中间体逐步形成 C-C 键与丙烯酸苄酯发生反应,生成环丙烷产物。通过MD模拟研究了酶中观察到的非对映选择性和对映选择性的起源,这表明通过空间控制优选形成顺式环丙烷。






























 京公网安备 11010802027423号
京公网安备 11010802027423号