当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Enantioselective C(sp3)−SCF3 Coupling of Carbon-Centered Benzyl Radicals with (Me4N)SCF3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-01-26 , DOI: 10.1002/anie.202319850
Wei Zhang 1, 2 , Yu Tian 1, 2 , Xiao-Dong Liu 1, 2 , Cheng Luan 1, 2 , Ji-Ren Liu 1, 2 , Qiang-Shuai Gu 3 , Zhong-Liang Li 4 , Xin-Yuan Liu 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-01-26 , DOI: 10.1002/anie.202319850
Wei Zhang 1, 2 , Yu Tian 1, 2 , Xiao-Dong Liu 1, 2 , Cheng Luan 1, 2 , Ji-Ren Liu 1, 2 , Qiang-Shuai Gu 3 , Zhong-Liang Li 4 , Xin-Yuan Liu 1, 2
Affiliation
|
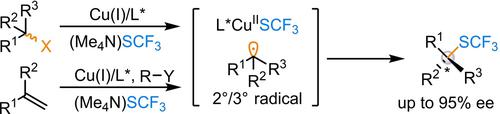
A copper-catalyzed enantioselective radical C(sp3)−SCF3 coupling of secondary/tertiary benzyl radicals with the easily available (Me4N)SCF3 reagent was developed to afford enantioenriched trifluoromethylthiolated molecules. The key to the success lies in the utilization of chiral phosphino-oxazoline-derived anionic N,N,P-ligands for the reaction initiation and enantioselectivity.
中文翻译:

铜催化碳中心苄基与 (Me4N)SCF3 的对映选择性 C(sp3)−SCF3 偶联
开发了铜催化的对映选择性自由基 C(sp 3 )−SCF 3仲/叔苄基自由基与容易获得的 (Me 4 N)SCF 3试剂的偶联,以提供对映体富集的三氟甲基硫醇化分子。成功的关键在于利用手性膦基恶唑啉衍生的阴离子N,N,P-配体来引发反应并提高对映选择性。
更新日期:2024-01-26
中文翻译:

铜催化碳中心苄基与 (Me4N)SCF3 的对映选择性 C(sp3)−SCF3 偶联
开发了铜催化的对映选择性自由基 C(sp 3 )−SCF 3仲/叔苄基自由基与容易获得的 (Me 4 N)SCF 3试剂的偶联,以提供对映体富集的三氟甲基硫醇化分子。成功的关键在于利用手性膦基恶唑啉衍生的阴离子N,N,P-配体来引发反应并提高对映选择性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号