Experimental Neurology ( IF 4.6 ) Pub Date : 2024-01-26 , DOI: 10.1016/j.expneurol.2024.114703
Ye Yuan 1 , Qiuguang He 1 , Xiao Yang 2 , Jerry J Flores 3 , Lei Huang 4 , Xu Luo 5 , Xingyu Zhang 5 , Zongyi Zhang 5 , Ruihao Li 5 , Lingui Gu 6 , Siyuan Dong 3 , Shiyi Zhu 3 , Kun Yi 7 , Mingyang Han 3 , Lei Wu 3 , You Zhou 1 , John H Zhang 8 , Zongyi Xie 5 , Jiping Tang 3
|
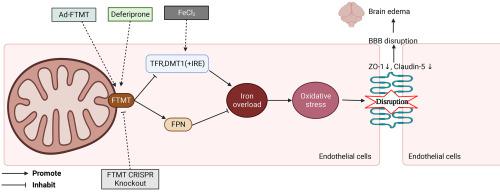
Germinal matrix hemorrhage (GMH) is a devasting neurological disease in premature newborns. After GMH, brain iron overload associated with hemoglobin degradation contributed to oxidative stress, causing disruption of the already vulnerable blood-brain barrier (BBB). Mitochondrial ferritin (FTMT), a novel mitochondrial outer membrane protein, is crucial in maintaining cellular iron homeostasis. We aimed to investigate the effect of FTMT upregulation on oxidative stress and BBB disruption associated with brain iron overload in rats. A total of 222 Sprague-Dawley neonatal rat pups (7 days old) were used to establish a collagenase-induced GMH model and an iron-overload model of intracerebral FeCl2 injection. Deferiprone was administered via gastric lavage 1 h after GMH and given daily until euthanasia. FTMT CRISPR Knockout and adenovirus (Ad)-FTMT were administered intracerebroventricularly 48 h before GMH and FeCl2 injection, respectively. Neurobehavioral tests, immunofluorescence, Western blot, Malondialdehyde measurement, and brain water content were performed to evaluate neurobehavior deficits, oxidative stress, and BBB disruption, respectively. The results demonstrated that brain expressions of iron exporter Ferroportin (FPN) and antioxidant glutathione peroxidase 4 (GPX4) as well as BBB tight junction proteins including Claudin-5 and Zona Occulta (ZO)-1 were found to be decreased at 72 h after GMH. FTMT agonist Deferiprone attenuated oxidative stress and preserved BBB tight junction proteins after GMH. These effects were partially reversed by FTMT CRISPR Knockout. Iron overload by FeCl2 injection resulted in oxidative stress and BBB disruption, which were improved by Ad-FTMT mediated FTMT overexpression. Collectively, FTMT upregulation is neuroprotective against brain injury associated with iron overload. Deferiprone reduced oxidative stress and BBB disruption by maintaining cellular iron homeostasis partially by the upregulating of FTMT after GMH. Deferiprone may be an effective treatment for patients with GMH.
中文翻译:

线粒体铁蛋白上调通过维持新生大鼠生发基质出血模型中的细胞铁稳态来减少氧化应激和血脑屏障破坏
生发基质出血(GMH)是早产儿的一种毁灭性神经系统疾病。 GMH 后,与血红蛋白降解相关的脑铁超载会导致氧化应激,导致本已脆弱的血脑屏障 (BBB) 受到破坏。线粒体铁蛋白(FTMT)是一种新型线粒体外膜蛋白,对于维持细胞铁稳态至关重要。我们的目的是研究 FTMT 上调对大鼠脑铁超载相关氧化应激和血脑屏障破坏的影响。采用222只Sprague-Dawley幼鼠(7日龄)建立胶原酶诱导的GMH模型和脑内注射FeCl 2铁超载模型。 GMH 后 1 小时通过洗胃给予去铁酮,并每天给予直至安乐死。分别在注射GMH和FeCl 2前48小时脑室内施用FTMT CRISPR Knockout和腺病毒(Ad)-FTMT 。分别进行神经行为测试、免疫荧光、蛋白质印迹、丙二醛测量和脑含水量来评估神经行为缺陷、氧化应激和血脑屏障破坏。结果表明,GMH 后 72 小时,大脑中铁输出蛋白 Ferroportin (FPN) 和抗氧化剂谷胱甘肽过氧化物酶 4 (GPX4) 以及 BBB 紧密连接蛋白(包括 Claudin-5 和 Zona Occulta (ZO)-1)的表达降低。 FTMT 激动剂去铁酮可减轻 GMH 后的氧化应激并保留 BBB 紧密连接蛋白。 FTMT CRISPR 敲除部分逆转了这些效应。 FeCl 2注射引起的铁过载会导致氧化应激和血脑屏障破坏,而 Ad-FTMT 介导的 FTMT 过表达可改善这些情况。总的来说,FTMT 上调具有神经保护作用,可预防与铁超载相关的脑损伤。去铁酮通过维持细胞铁稳态来减少氧化应激和 BBB 破坏,部分原因是 GMH 后 FTMT 上调。去铁酮可能是 GMH 患者的有效治疗方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号