当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereospecific Deoxygenation of Aliphatic Epoxides to Alkenes under Rhenium Catalysis
Organic Letters ( IF 4.9 ) Pub Date : 2015-06-12 00:00:00 , DOI: 10.1021/acs.orglett.5b01583
Takuya Nakagiri 1 , Masahito Murai 1 , Kazuhiko Takai 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2015-06-12 00:00:00 , DOI: 10.1021/acs.orglett.5b01583
Takuya Nakagiri 1 , Masahito Murai 1 , Kazuhiko Takai 1, 2
Affiliation
|
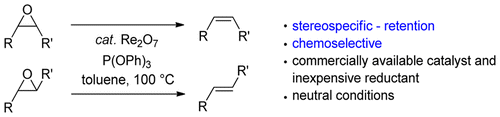
The combination of a catalytic amount of Re2O7 and triphenyl phosphite as a reductant is effective for the deoxygenation of unactivated aliphatic epoxides to alkenes. The reaction proceeds stereospecifically with variously substituted epoxides under neutral conditions and is compatible with various functional groups. Protection and deprotection of a double bond functionality using an epoxide are shown as an example of the current rhenium-catalyzed deoxygenation protocol. The effect of reductants for the stereoselectivity has also been studied, indicating that the use of electron-deficient phosphines or phosphites is the key for the stereospecific deoxygenation.
中文翻译:

R催化下脂环氧化物的立体定向脱氧为烯烃
催化量的Re 2 O 7和亚磷酸三苯酯作为还原剂的组合对于未活化的脂族环氧化物脱氧为烯烃是有效的。该反应在中性条件下与各种取代的环氧化物立体定向进行,并且与各种官能团相容。作为当前rh催化的脱氧方案的实例,显示了使用环氧化物对双键官能团的保护和脱保护。还研究了还原剂对立体选择性的影响,表明使用缺电子的膦或亚磷酸酯是立体特异性脱氧的关键。
更新日期:2015-06-12
中文翻译:

R催化下脂环氧化物的立体定向脱氧为烯烃
催化量的Re 2 O 7和亚磷酸三苯酯作为还原剂的组合对于未活化的脂族环氧化物脱氧为烯烃是有效的。该反应在中性条件下与各种取代的环氧化物立体定向进行,并且与各种官能团相容。作为当前rh催化的脱氧方案的实例,显示了使用环氧化物对双键官能团的保护和脱保护。还研究了还原剂对立体选择性的影响,表明使用缺电子的膦或亚磷酸酯是立体特异性脱氧的关键。

































 京公网安备 11010802027423号
京公网安备 11010802027423号