当前位置:
X-MOL 学术
›
Chem Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective direct deuterodecarboxylation of free aliphatic carboxylic acids enabled by deuteron-coupled electron transfer
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-01-24 , DOI: 10.1016/j.checat.2023.100899 Chen-Qiang Deng , Yuantai Xu , Jia-Hao Luo , Guang-Zu Wang , Jin Deng , Yao Fu
Chem Catalysis ( IF 11.5 ) Pub Date : 2024-01-24 , DOI: 10.1016/j.checat.2023.100899 Chen-Qiang Deng , Yuantai Xu , Jia-Hao Luo , Guang-Zu Wang , Jin Deng , Yao Fu
|
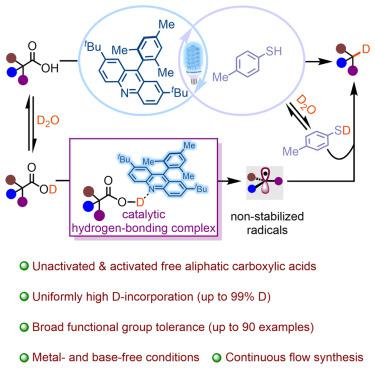
Decarboxylative deuteration of activated precursors (e.g., -amino acids) is an effective method for installing deuterium atoms at activated positions; however, direct usage of unactivated aliphatic carboxylic acids remains a long-standing challenge for the construction of C(sp)–D bonds at non-activated positions. Herein, we report a general and efficient photoinduced direct deuterodecarboxylation of free aliphatic carboxylic acids via synergistic acridine and thiophenol catalyst systems with DO as the cost-effective deuterium source, achieving precise installation of a deuterium atom at non-activated positions. The present methodology features broad functional group tolerance and displays high chemoselectivity, enabling the late-stage deuteration of natural products and pharmaceuticals, with high levels of D-incorporation (up to 90 examples, up to 99% D). Moreover, a 50 mmol scale-up reaction in the continuous flow process has been demonstrated without loss of D-incorporation and greatly improves the production efficiency with a shortened reaction time from 12 h to 15 min.
中文翻译:

通过氘核耦合电子转移实现游离脂肪族羧酸的化学选择性直接氘代脱羧
活化前体(例如α-氨基酸)的脱羧氘化是在活化位置安装氘原子的有效方法;然而,直接使用未活化的脂肪族羧酸对于在非活化位置构建 C(sp)-D 键仍然是一个长期的挑战。在此,我们报道了通过吖啶和苯硫酚协同催化剂体系,以 DO 作为经济高效的氘源,对游离脂肪族羧酸进行通用且高效的光诱导直接氘脱羧,实现了氘原子在非活化位置的精确安装。本方法具有广泛的官能团耐受性和高化学选择性,能够对天然产物和药物进行后期氘化,并具有高水平的 D-掺入(最多 90 个示例,高达 99% D)。此外,连续流动过程中的50 mmol放大反应已被证明不会损失D-掺入,并且大大提高了生产效率,反应时间从12小时缩短至15分钟。
更新日期:2024-01-24
中文翻译:

通过氘核耦合电子转移实现游离脂肪族羧酸的化学选择性直接氘代脱羧
活化前体(例如α-氨基酸)的脱羧氘化是在活化位置安装氘原子的有效方法;然而,直接使用未活化的脂肪族羧酸对于在非活化位置构建 C(sp)-D 键仍然是一个长期的挑战。在此,我们报道了通过吖啶和苯硫酚协同催化剂体系,以 DO 作为经济高效的氘源,对游离脂肪族羧酸进行通用且高效的光诱导直接氘脱羧,实现了氘原子在非活化位置的精确安装。本方法具有广泛的官能团耐受性和高化学选择性,能够对天然产物和药物进行后期氘化,并具有高水平的 D-掺入(最多 90 个示例,高达 99% D)。此外,连续流动过程中的50 mmol放大反应已被证明不会损失D-掺入,并且大大提高了生产效率,反应时间从12小时缩短至15分钟。






























 京公网安备 11010802027423号
京公网安备 11010802027423号