当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Various Acid Anions on Potassium Poisoning and the Mechanism of Commercial V2O5–WO3/TiO2 Catalysts for SCR of NOx
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-01-24 , DOI: 10.1021/acs.iecr.3c03785
Jianyi Zhang 1 , Jifa Miao 1, 2 , Biyi Huang 1 , Yanting Chen 1, 2 , Jinsheng Chen 1, 2 , Jinxiu Wang 1, 2
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-01-24 , DOI: 10.1021/acs.iecr.3c03785
Jianyi Zhang 1 , Jifa Miao 1, 2 , Biyi Huang 1 , Yanting Chen 1, 2 , Jinsheng Chen 1, 2 , Jinxiu Wang 1, 2
Affiliation
|
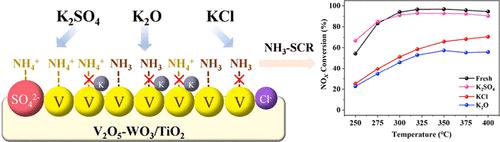
The poisoning of K2O, KCl, and K2SO4 on commercial V2O5–WO3/TiO2 catalysts used for selective catalytic reduction (SCR) of NOx by NH3 was investigated, respectively. The degree of inactivation observed in these catalysts poisoned with different forms of potassium follows K2O ≈ KCl > K2SO4. At 350 °C, the NOx conversion of the K2SO4 poisoning catalyst (0.5 wt % K) was 53%, while that of the K2O and KCl poisoning catalysts was only 2 and 4%, respectively. The atomic ratio of V5+/V4+ (0.76) and the proportion of adsorbed oxygen species (48%) on the surface of the K2SO4 poisoning sample were higher than those of the K2O poisoning sample (0.64, 36%) and the KCl poisoning sample (0.66, 36%). The above phenomena were due to the different poisoning mechanisms caused by K2O, KCl, and K2SO4. K2O decreased the number of Brønsted and Lewis acid sites and, moreover, reduced V5+ to V4+; K in KCl preferred to occupy Brønsted acid sites and Cl in KCl reduced Lewis acid sites. SO42– in K2SO4 counteracted the impact of K on the surface acid sites and V5+ of the poisoned catalyst and generated new Brønsted acid sites.
中文翻译:

不同酸阴离子对钾中毒的影响及商用V2O5-WO3/TiO2催化剂用于NOx SCR的机理
分别研究了K 2 O、KCl和K 2 SO 4对用于NH 3选择性催化还原(SCR)NO x的商业V 2 O 5 –WO 3 /TiO 2催化剂的中毒情况。在这些被不同形式的钾中毒的催化剂中观察到的失活程度遵循K 2 O ≈ KCl > K 2 SO 4。在350℃下,K 2 SO 4中毒催化剂(0.5 wt% K)的NO x转化率为53%,而K 2 O和KCl中毒催化剂的NO x 转化率分别仅为2%和4%。 K 2 SO 4中毒样品表面的V 5+ /V 4+原子比(0.76)和吸附氧比例(48%)均高于K 2 O中毒样品(0.64, 36%) 和 KCl 中毒样品 (0.66, 36%)。上述现象是由于K 2 O、KCl、K 2 SO 4引起的中毒机理不同所致。 K 2 O 减少了 Brønsted 和 Lewis 酸性位点的数量,此外,还将 V 5+还原为 V 4+; KCl 中的 K 优先占据布朗斯台德酸位,而 KCl 中的 Cl 则优先占据路易斯酸位。K 2 SO 4中的SO 4 2–抵消了K对中毒催化剂表面酸位和V 5+的影响,并产生了新的布朗斯台德酸位。
更新日期:2024-01-24
中文翻译:

不同酸阴离子对钾中毒的影响及商用V2O5-WO3/TiO2催化剂用于NOx SCR的机理
分别研究了K 2 O、KCl和K 2 SO 4对用于NH 3选择性催化还原(SCR)NO x的商业V 2 O 5 –WO 3 /TiO 2催化剂的中毒情况。在这些被不同形式的钾中毒的催化剂中观察到的失活程度遵循K 2 O ≈ KCl > K 2 SO 4。在350℃下,K 2 SO 4中毒催化剂(0.5 wt% K)的NO x转化率为53%,而K 2 O和KCl中毒催化剂的NO x 转化率分别仅为2%和4%。 K 2 SO 4中毒样品表面的V 5+ /V 4+原子比(0.76)和吸附氧比例(48%)均高于K 2 O中毒样品(0.64, 36%) 和 KCl 中毒样品 (0.66, 36%)。上述现象是由于K 2 O、KCl、K 2 SO 4引起的中毒机理不同所致。 K 2 O 减少了 Brønsted 和 Lewis 酸性位点的数量,此外,还将 V 5+还原为 V 4+; KCl 中的 K 优先占据布朗斯台德酸位,而 KCl 中的 Cl 则优先占据路易斯酸位。K 2 SO 4中的SO 4 2–抵消了K对中毒催化剂表面酸位和V 5+的影响,并产生了新的布朗斯台德酸位。

































 京公网安备 11010802027423号
京公网安备 11010802027423号