当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intense Left-handed Circularly Polarized Luminescence in Chiral Nematic Hydroxypropyl Cellulose Composite Films
Advanced Materials ( IF 27.4 ) Pub Date : 2024-01-25 , DOI: 10.1002/adma.202308742 Yuxiao Huang 1 , Yi Qian 1 , Yongxin Chang 2 , Jiaqi Yu 1 , Qiongya Li 2 , Mingliang Tang 3 , Xindi Yang 2 , Zhepai Liu 1 , Hui Li 1 , Zece Zhu 1 , Wei Li 1 , Fusheng Zhang 1, 2 , Guangyan Qing 1, 2
Advanced Materials ( IF 27.4 ) Pub Date : 2024-01-25 , DOI: 10.1002/adma.202308742 Yuxiao Huang 1 , Yi Qian 1 , Yongxin Chang 2 , Jiaqi Yu 1 , Qiongya Li 2 , Mingliang Tang 3 , Xindi Yang 2 , Zhepai Liu 1 , Hui Li 1 , Zece Zhu 1 , Wei Li 1 , Fusheng Zhang 1, 2 , Guangyan Qing 1, 2
Affiliation
|
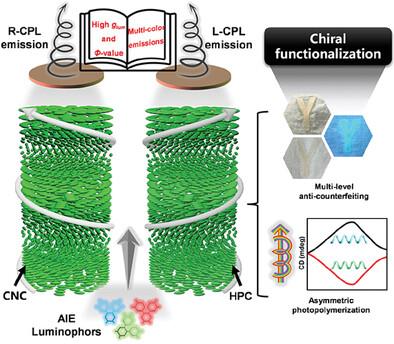
Integrating optically active components into chiral photonic cellulose to fabricate circularly polarized luminescent materials has transformative potential in disease detection, asymmetric reactions, and anticounterfeiting techniques. However, the lack of cellulose-based left-handed circularly polarized light (L-CPL) emissions hampers the progress of these chiral functionalizations. Here, this work proposes an unprecedented strategy: incorporating a chiral nematic organization of hydroxypropyl cellulose with robust aggregation-induced emission luminogens to generate intense L-CPL emission. By utilizing N,N-dimethylformamide as a good solvent for fluorescent components and cellulose matrices, this work produces a right-handed chiral nematic structure film with a uniform appearance in reflective and fluorescent states. Remarkably, this system integrates a high asymmetric factor (0.51) and an impressive emission quantum yield (55.8%) into one fascinating composite. More meaningfully, this approach is versatile, allowing for the incorporation of luminogen derivatives emitting multicolored L-CPL. These chiral fluorescent films possess exceptional mechanical flexibility (toughness up to 0.9 MJ m−3) and structural stability even under harsh environmental exposures, making them promising for the fabrication of various products. Additionally, these films can be cast on the fabrics to reveal multilevel and durable anticounterfeiting capabilities or used as a chiral light source to induce enantioselective photopolymerization, thereby offering significant potential for diverse practical applications.
中文翻译:

手性向列型羟丙基纤维素复合薄膜中的强左手圆偏振发光
将光学活性成分集成到手性光子纤维素中以制造圆偏振发光材料,在疾病检测、不对称反应和防伪技术方面具有变革潜力。然而,缺乏基于纤维素的左手圆偏振光(L-CPL)发射阻碍了这些手性官能化的进展。在这里,这项工作提出了一种前所未有的策略:将羟丙基纤维素的手性向列组织与强大的聚集诱导发射发光体相结合,以产生强烈的 L-CPL 发射。该工作利用N , N-二甲基甲酰胺作为荧光成分和纤维素基质的良好溶剂,制备了在反射态和荧光态下均具有均匀外观的右手向列结构薄膜。值得注意的是,该系统将高不对称因子 (0.51) 和令人印象深刻的发射量子产率 (55.8%) 集成到一种令人着迷的复合材料中。更有意义的是,这种方法是通用的,允许掺入发射多色 L-CPL 的发光体衍生物。这些手性荧光薄膜即使在恶劣的环境下也具有出色的机械柔韧性(韧性高达0.9 MJ m -3 )和结构稳定性,使其有望用于制造各种产品。此外,这些薄膜可以浇铸在织物上,以展现多层次和持久的防伪能力,或用作手性光源来诱导对映选择性光聚合,从而为各种实际应用提供巨大的潜力。
更新日期:2024-01-25
中文翻译:

手性向列型羟丙基纤维素复合薄膜中的强左手圆偏振发光
将光学活性成分集成到手性光子纤维素中以制造圆偏振发光材料,在疾病检测、不对称反应和防伪技术方面具有变革潜力。然而,缺乏基于纤维素的左手圆偏振光(L-CPL)发射阻碍了这些手性官能化的进展。在这里,这项工作提出了一种前所未有的策略:将羟丙基纤维素的手性向列组织与强大的聚集诱导发射发光体相结合,以产生强烈的 L-CPL 发射。该工作利用N , N-二甲基甲酰胺作为荧光成分和纤维素基质的良好溶剂,制备了在反射态和荧光态下均具有均匀外观的右手向列结构薄膜。值得注意的是,该系统将高不对称因子 (0.51) 和令人印象深刻的发射量子产率 (55.8%) 集成到一种令人着迷的复合材料中。更有意义的是,这种方法是通用的,允许掺入发射多色 L-CPL 的发光体衍生物。这些手性荧光薄膜即使在恶劣的环境下也具有出色的机械柔韧性(韧性高达0.9 MJ m -3 )和结构稳定性,使其有望用于制造各种产品。此外,这些薄膜可以浇铸在织物上,以展现多层次和持久的防伪能力,或用作手性光源来诱导对映选择性光聚合,从而为各种实际应用提供巨大的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号