当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of the 1,3-Dienyl-5-Alkyl-6-Oxy Motif: Method Development and Total Synthesis
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-01-25 , DOI: 10.1002/anie.202400478 Jie Wang 1 , Chuning Guo 1 , Yaqian Liu 1 , Yunpeng Ji 1 , Hongli Jia 1 , Houhua Li 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2024-01-25 , DOI: 10.1002/anie.202400478 Jie Wang 1 , Chuning Guo 1 , Yaqian Liu 1 , Yunpeng Ji 1 , Hongli Jia 1 , Houhua Li 1
Affiliation
|
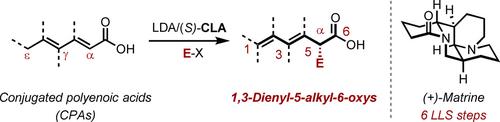
Method development and total synthesis have resulted in the enantioselective synthesis of the 1,3-dienyl-5-alkyl-6-oxy motif, and thus expedient total syntheses of three types of natural products (glutarimide antibiotics, α-pyrone polyketides and Lupin alkaloids), completed within 4–7 steps.
中文翻译:

1,3-二烯基-5-烷基-6-氧基基序的对映选择性合成:方法开发和全合成
方法开发和全合成实现了1,3-二烯基-5-烷基-6-氧基的对映选择性合成,从而方便地全合成了三种天然产物(戊二酰亚胺抗生素、α-吡喃酮聚酮化合物和羽扇豆生物碱) ),在 4-7 个步骤内完成。
更新日期:2024-01-25
中文翻译:

1,3-二烯基-5-烷基-6-氧基基序的对映选择性合成:方法开发和全合成
方法开发和全合成实现了1,3-二烯基-5-烷基-6-氧基的对映选择性合成,从而方便地全合成了三种天然产物(戊二酰亚胺抗生素、α-吡喃酮聚酮化合物和羽扇豆生物碱) ),在 4-7 个步骤内完成。





























 京公网安备 11010802027423号
京公网安备 11010802027423号