当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Molecular Cage Accessed by Threefold Click Reaction of a C3v-Symmetric Triazido-Functionalized Tribenzotriquinacene
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-01-25 , DOI: 10.1021/acs.joc.3c01349 Shuai-Nan Liu 1 , Qing-Xia Ren 1 , Yun-Tao Ding 1 , Xiao-Ping Cao 1 , Zi-Fa Shi 1 , Hak-Fun Chow 2 , Dietmar Kuck 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-01-25 , DOI: 10.1021/acs.joc.3c01349 Shuai-Nan Liu 1 , Qing-Xia Ren 1 , Yun-Tao Ding 1 , Xiao-Ping Cao 1 , Zi-Fa Shi 1 , Hak-Fun Chow 2 , Dietmar Kuck 3
Affiliation
|
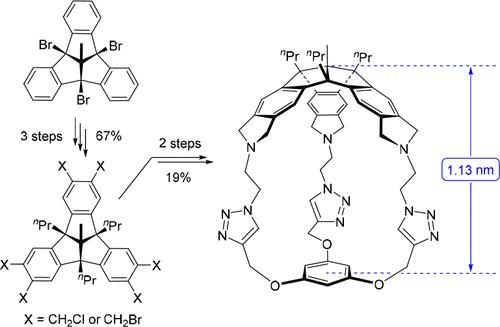
The hitherto unknown hexakis(halomethyl)-functionalized tribenzotriquinacenes (TBTQs) 9 and 10 were synthesized from the key 4b,8b,12b-tribromo-TBTQ derivative 6 by an improved route in 67% overall yield. Extension of the bowl-shaped framework of 9 or 10 by threefold condensation with propargylamine or 2-azidoethylamine afforded the corresponding TBTQ-trialkyne 11 and TBTQ-triazide 12, respectively. While attempts to construct bis-TBTQ cages, including homodimerization of 11 and heterocoupling of 11 with 12, were unsuccessful, triazide 12 was found to undergo threefold [3 + 2]-cycloaddition with 3-ethynylaniline and phloroglucinol tripropargyl ether under click chemistry conditions. The latter reaction enabled facile capping of the TBTQ bowl to give the novel cage compound 5 in 22% yield.
中文翻译:

通过 C3v 对称三叠氮基官能化三苯并三醌的三重点击反应进入分子笼
由关键的4b,8b,12b-三溴-TBTQ衍生物6通过改进的路线合成了迄今为止未知的六(卤甲基)官能化三苯并三醌(TBTQ) 9和10 ,总产率为67%。 9或10的碗状骨架通过与炔丙胺或2-叠氮乙胺三倍缩合而延伸,分别得到相应的TBTQ-三炔11和TBTQ-三叠氮化物12 。虽然构建双-TBTQ笼的尝试,包括11的同二聚化和11与12的杂偶联均未成功,但发现三叠氮12在点击化学条件下与3-乙炔基苯胺和间苯三酚三炔丙基醚发生三重[3+2]-环加成。后一个反应能够轻松封盖 TBTQ 碗,以 22% 的产率得到新型笼状化合物5 。
更新日期:2024-01-25
中文翻译:

通过 C3v 对称三叠氮基官能化三苯并三醌的三重点击反应进入分子笼
由关键的4b,8b,12b-三溴-TBTQ衍生物6通过改进的路线合成了迄今为止未知的六(卤甲基)官能化三苯并三醌(TBTQ) 9和10 ,总产率为67%。 9或10的碗状骨架通过与炔丙胺或2-叠氮乙胺三倍缩合而延伸,分别得到相应的TBTQ-三炔11和TBTQ-三叠氮化物12 。虽然构建双-TBTQ笼的尝试,包括11的同二聚化和11与12的杂偶联均未成功,但发现三叠氮12在点击化学条件下与3-乙炔基苯胺和间苯三酚三炔丙基醚发生三重[3+2]-环加成。后一个反应能够轻松封盖 TBTQ 碗,以 22% 的产率得到新型笼状化合物5 。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号