当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DNA-Mediated Self-Organization of Polymeric Nanocompartments Leads to Interconnected Artificial Organelles
Nano Letters ( IF 9.6 ) Pub Date : 2016-10-17 00:00:00 , DOI: 10.1021/acs.nanolett.6b03430
Juan Liu 1 , Viktoriia Postupalenko 1 , Samuel Lörcher 1 , Dalin Wu 1 , Mohamed Chami 2 , Wolfgang Meier 1 , Cornelia G. Palivan 1
Nano Letters ( IF 9.6 ) Pub Date : 2016-10-17 00:00:00 , DOI: 10.1021/acs.nanolett.6b03430
Juan Liu 1 , Viktoriia Postupalenko 1 , Samuel Lörcher 1 , Dalin Wu 1 , Mohamed Chami 2 , Wolfgang Meier 1 , Cornelia G. Palivan 1
Affiliation
|
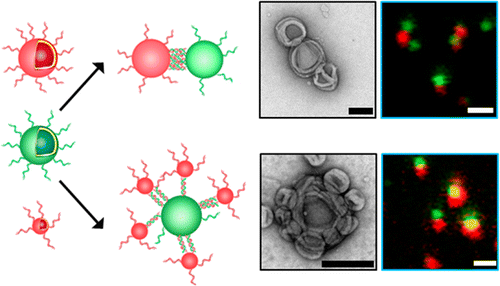
Self-organization of nanocomponents was mainly focused on solid nanoparticles, quantum dots, or liposomes to generate complex architectures with specific properties, but intrinsically limited or not developed enough, to mimic sophisticated structures with biological functions in cells. Here, we present a biomimetic strategy to self-organize synthetic nanocompartments (polymersomes) into clusters with controlled properties and topology by exploiting DNA hybridization to interconnect polymersomes. Molecular and external factors affecting the self-organization served to design clusters mimicking the connection of natural organelles: fine-tune of the distance between tethered polymersomes, different topologies, no fusion of clustered polymersomes, and no aggregation. Unexpected, extended DNA bridges that result from migration of the DNA strands inside the thick polymer membrane (about 12 nm) represent a key stability and control factor, not yet exploited for other synthetic nano-object networks. The replacement of the empty polymersomes with artificial organelles, already reported for single polymersome architecture, will provide an excellent platform for the development of artificial systems mimicking natural organelles or cells and represents a fundamental step in the engineering of molecular factories.
中文翻译:

DNA介导的聚合物纳米隔室的自组织导致相互连接的人工细胞器。
纳米组分的自组织主要集中在固体纳米颗粒,量子点或脂质体上,以产生具有特定性质的复杂结构,但本质上有限或不够发达,无法模仿细胞中具有生物学功能的复杂结构。在这里,我们提出了一种仿生策略,通过利用DNA杂交来互连聚合物囊泡,将合成的纳米隔室(聚合物囊泡)自组织成具有受控特性和拓扑的簇。影响自组织的分子和外部因素有助于设计模仿天然细胞器连接的簇:对连接的聚合物小体之间的距离进行微调,不同的拓扑结构,没有簇状的聚合物小体融合以及没有聚集。意外,由DNA链在厚聚合物膜(约12 nm)内迁移导致的延伸DNA桥代表了关键的稳定性和控制因素,尚未被其他合成纳米物体网络所利用。用单细胞体结构替代人工细胞器来代替空的聚合物小体,将为开发模仿天然细胞器或细胞的人工系统提供一个极好的平台,并且代表了分子工厂工程中的基础性步骤。
更新日期:2016-10-17
中文翻译:

DNA介导的聚合物纳米隔室的自组织导致相互连接的人工细胞器。
纳米组分的自组织主要集中在固体纳米颗粒,量子点或脂质体上,以产生具有特定性质的复杂结构,但本质上有限或不够发达,无法模仿细胞中具有生物学功能的复杂结构。在这里,我们提出了一种仿生策略,通过利用DNA杂交来互连聚合物囊泡,将合成的纳米隔室(聚合物囊泡)自组织成具有受控特性和拓扑的簇。影响自组织的分子和外部因素有助于设计模仿天然细胞器连接的簇:对连接的聚合物小体之间的距离进行微调,不同的拓扑结构,没有簇状的聚合物小体融合以及没有聚集。意外,由DNA链在厚聚合物膜(约12 nm)内迁移导致的延伸DNA桥代表了关键的稳定性和控制因素,尚未被其他合成纳米物体网络所利用。用单细胞体结构替代人工细胞器来代替空的聚合物小体,将为开发模仿天然细胞器或细胞的人工系统提供一个极好的平台,并且代表了分子工厂工程中的基础性步骤。































 京公网安备 11010802027423号
京公网安备 11010802027423号