当前位置:
X-MOL 学术
›
Results Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of new N-alkylated 6-bromoindoline-2.3-dione derivatives: Crystal structures, spectroscopic characterizations, Hirschfeld surface analyses, molecular docking studies, DFT calculations, and antibacterial activity
Results in Chemistry ( IF 2.5 ) Pub Date : 2024-01-24 , DOI: 10.1016/j.rechem.2024.101338
Nohaila Rharmili , Yusuf Sert , Youssef Kandri Rodi , Fouad Ouazzani Chahdi , Amal Haoudi , Joel T. Mague , Ahmed Mazzah , Naoufal El Hachlafi , Nesrine Benkhaira , Kawtar Fikri-Benbrahim , El Mokhtar Essassi , Nada Kheira Sebbar
Results in Chemistry ( IF 2.5 ) Pub Date : 2024-01-24 , DOI: 10.1016/j.rechem.2024.101338
Nohaila Rharmili , Yusuf Sert , Youssef Kandri Rodi , Fouad Ouazzani Chahdi , Amal Haoudi , Joel T. Mague , Ahmed Mazzah , Naoufal El Hachlafi , Nesrine Benkhaira , Kawtar Fikri-Benbrahim , El Mokhtar Essassi , Nada Kheira Sebbar
|
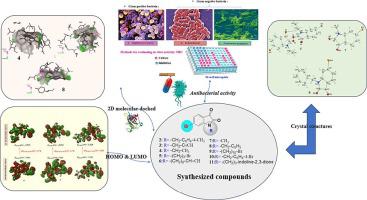
Ten new isatin derivatives (2–11) were synthesized by alkylation reactions under phase transfer catalysis (PTC) conditions. Structural characterization of all compounds was performed using H and C NMR spectroscopy. The molecular and crystal structures of three compounds (4, 7, and 8) were confirmed through single-crystal X-ray diffraction analysis. Spectral data were also calculated using density function theory (DFT) at the B3LYP/6–311++G(d, p) level and compared to the experimental results, to understand the non-binding intermolecular interactions in the solid phase crystal packing. The closest contacts between atoms of the three molecules studied were identified by 2D and 3D Hirshfeld surface analyses. The structures of 4, 7 and 8 are optimized and their HOMO and LUMO energies, as well as their orbital pictures were evaluated. The experimental results correlated well with the calculated results. Finally, molecular docking studies of 4, 7 and 8 were performed to investigate their binding patterns with inhibitory targets from the Protein Data Bank (PDB: 3G7B and PDB: 3T0T using the AutoDock Vina program. The antibacterial activities of 2–11 against Gram-positive and Gram-negative microbial strains, such as were evaluated, and the findings indicated that 4 and 8 exhibited the highest efficacy, as reflected in their minimal inhibitory concentration (MIC) values.
中文翻译:

新型 N-烷基化 6-溴二氢吲哚-2.3-二酮衍生物的合成:晶体结构、光谱表征、Hirschfeld 表面分析、分子对接研究、DFT 计算和抗菌活性
在相转移催化(PTC)条件下通过烷基化反应合成了十种新的靛红衍生物(2-11)。所有化合物的结构表征均使用 1H 和 13C NMR 光谱进行。通过单晶X射线衍射分析证实了三种化合物(4、7和8)的分子和晶体结构。还使用密度函数理论 (DFT) 在 B3LYP/6–311++G(d, p) 水平上计算光谱数据,并与实验结果进行比较,以了解固相晶体堆积中的非结合分子间相互作用。通过 2D 和 3D Hirshfeld 表面分析确定了所研究的三个分子的原子之间最紧密的接触。对4、7和8的结构进行了优化,并评估了它们的HOMO和LUMO能量以及它们的轨道图。实验结果与计算结果吻合良好。最后,使用 AutoDock Vina 程序对 4、7 和 8 进行分子对接研究,以研究它们与蛋白质数据库(PDB:3G7B 和 PDB:3T0T)的抑制靶标的结合模式。2-11 对革兰氏阴性菌的抗菌活性对阳性和革兰氏阴性微生物菌株进行了评估,结果表明 4 和 8 表现出最高的功效,这反映在它们的最小抑菌浓度 (MIC) 值上。
更新日期:2024-01-24
中文翻译:

新型 N-烷基化 6-溴二氢吲哚-2.3-二酮衍生物的合成:晶体结构、光谱表征、Hirschfeld 表面分析、分子对接研究、DFT 计算和抗菌活性
在相转移催化(PTC)条件下通过烷基化反应合成了十种新的靛红衍生物(2-11)。所有化合物的结构表征均使用 1H 和 13C NMR 光谱进行。通过单晶X射线衍射分析证实了三种化合物(4、7和8)的分子和晶体结构。还使用密度函数理论 (DFT) 在 B3LYP/6–311++G(d, p) 水平上计算光谱数据,并与实验结果进行比较,以了解固相晶体堆积中的非结合分子间相互作用。通过 2D 和 3D Hirshfeld 表面分析确定了所研究的三个分子的原子之间最紧密的接触。对4、7和8的结构进行了优化,并评估了它们的HOMO和LUMO能量以及它们的轨道图。实验结果与计算结果吻合良好。最后,使用 AutoDock Vina 程序对 4、7 和 8 进行分子对接研究,以研究它们与蛋白质数据库(PDB:3G7B 和 PDB:3T0T)的抑制靶标的结合模式。2-11 对革兰氏阴性菌的抗菌活性对阳性和革兰氏阴性微生物菌株进行了评估,结果表明 4 和 8 表现出最高的功效,这反映在它们的最小抑菌浓度 (MIC) 值上。

































 京公网安备 11010802027423号
京公网安备 11010802027423号