当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrophilic Cyclization of Aryl Propargylic Alcohols: Synthesis of Dihalogenated 6,9-Dihydropyrido[1,2-a]indoles via a Cascade Iodocyclization
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-10-17 00:00:00 , DOI: 10.1021/acs.joc.6b02013 Jia Wang 1 , Hai-Tao Zhu 2 , Si Chen 1 , Yu Xia 1 , Dong-Po Jin 1 , Yi-Feng Qiu 1 , Ying-Xiu Li 1 , Yong-Min Liang 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2016-10-17 00:00:00 , DOI: 10.1021/acs.joc.6b02013 Jia Wang 1 , Hai-Tao Zhu 2 , Si Chen 1 , Yu Xia 1 , Dong-Po Jin 1 , Yi-Feng Qiu 1 , Ying-Xiu Li 1 , Yong-Min Liang 1
Affiliation
|
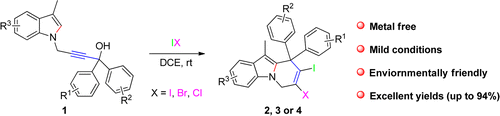
A strategy for the synthesis of 6,9-dihydropyrido[1,2-a]indoles through a cascade iodocyclization of 4-(3-methyl-1H-indol-1-yl)-1,1-diphenylbut-2-yn-1-ol derivatives is presented. This reaction was conducted under very mild conditions and in a short time. The reactions are metal-free, are environmentally friendly and give up to 94% yield. Moreover, the obtained halides allow functional group diversification by palladium-catalyzed coupling reactions, which could act as potential intermediates for the synthesis of valuable compounds.
中文翻译:

芳基丙炔醇的亲电环化:级联碘环化合成二卤代6,9-二氢吡啶并[1,2- a ]吲哚
通过4-(3-甲基-1 H-吲哚-1-基)-1,1-二苯基丁-2-yn的级联碘化反应合成6,9-二氢吡啶并[1,2- a ]吲哚的策略给出了-1-醇衍生物。该反应在非常温和的条件下并且在短时间内进行。该反应不含金属,对环境无害,产率高达94%。而且,所获得的卤化物允许通过钯催化的偶联反应使官能团多样化,其可以用作合成有价值的化合物的潜在中间体。
更新日期:2016-10-17
中文翻译:

芳基丙炔醇的亲电环化:级联碘环化合成二卤代6,9-二氢吡啶并[1,2- a ]吲哚
通过4-(3-甲基-1 H-吲哚-1-基)-1,1-二苯基丁-2-yn的级联碘化反应合成6,9-二氢吡啶并[1,2- a ]吲哚的策略给出了-1-醇衍生物。该反应在非常温和的条件下并且在短时间内进行。该反应不含金属,对环境无害,产率高达94%。而且,所获得的卤化物允许通过钯催化的偶联反应使官能团多样化,其可以用作合成有价值的化合物的潜在中间体。






























 京公网安备 11010802027423号
京公网安备 11010802027423号